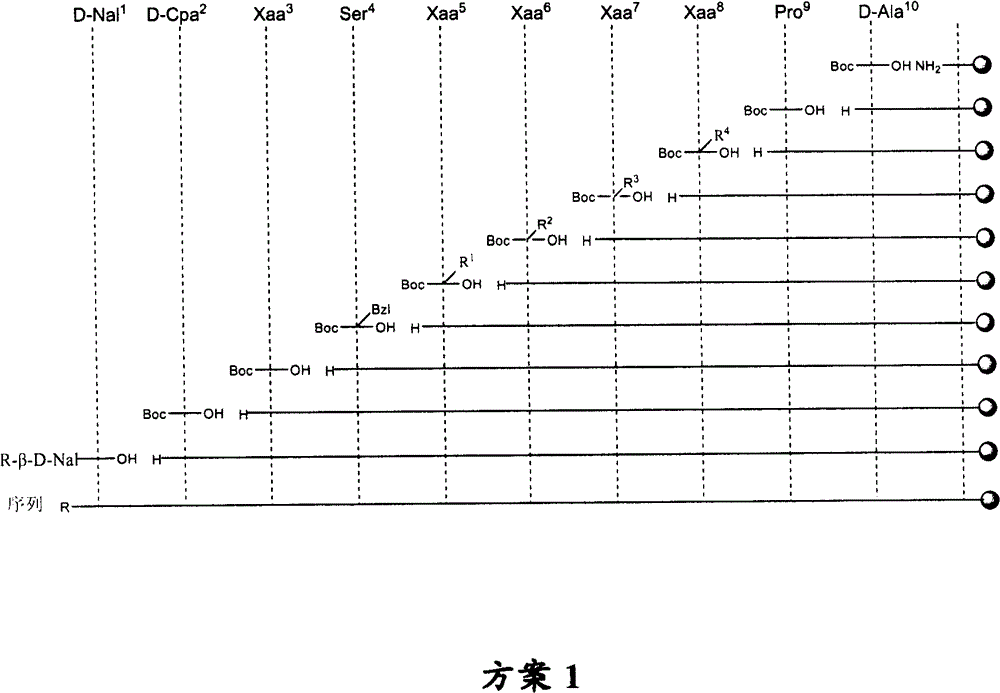
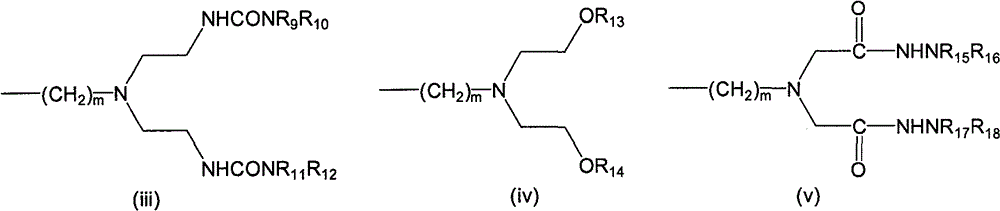
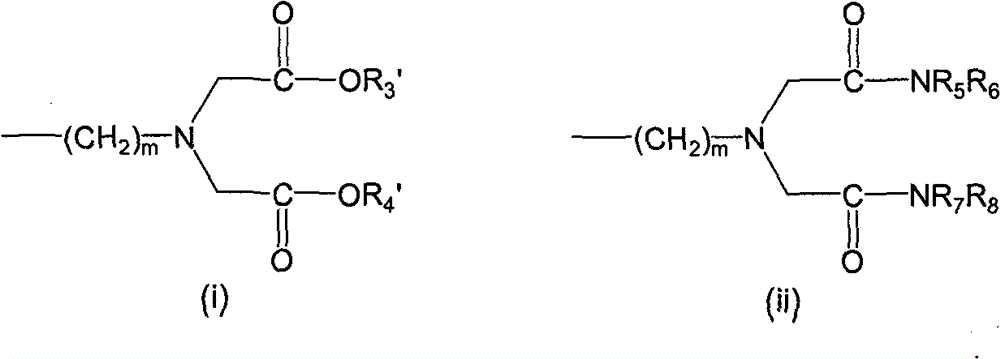LHRH antagonist with long-acting low-histamine release side effect
A CH2, R1O technology, applied in the field of decapeptide derivatives, can solve the problems of short half-life, low bioavailability, and high release of histamine, and achieve good antagonistic activity, low side effects of histamine release, and long in vivo action time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0388]Example 1: N, N-(dibenzyloxycarbonylmethyl)-aminoacetic acid [NTA B ]Synthesis
[0389] Dibenzyl iminodiacetate (28.8mmol, 9g) and bromoacetic acid (14.4mmol, 2.0g) were placed in a 100ml round bottom flask, and 50mL of ethanol was added to dissolve it. After adding TEA (10mL, 72mmol) in an ice bath, stir at room temperature for 72 hours, spin off the ethanol, adjust the pH of the aqueous solution to alkaline, wash with ether and then adjust the pH of the aqueous phase to acidic, extract with ethyl acetate, wash twice with water, and the ester layer Dry over anhydrous sodium sulfate. The ester layer was concentrated, and the oil was subjected to column chromatography to obtain 2 g of the oil, with a yield of 44.4%. TLC detection: chloroform:methanol:HOAc (20:1:0.5), Rf=0.3.
Embodiment 2
[0390] Embodiment 2: the synthesis of N-tert-butoxycarbonyl-imine-diacetic acid
[0391] 8.6g (65.6mmol) amino diacetic acid, add 80mL water, 40mL 2N NaOH, 80mL dioxane. Control pH=9, add dropwise 17.2g (Boc) 2 O (78.7 mmol, dissolved in 30 mL of dioxane). During the reaction process, the pH was kept at 9. After 12 hours, the dioxane was removed under reduced pressure. The aqueous phase was extracted with ether, acidified to pH 3 with citric acid, extracted 3 times with ethyl acetate and 3 times with chloroform, and then dried. Filtration and concentration gave 10.5 g of white solid. Yield 69.1%, Rf=0.56 (n-butanol:acetic acid:water, 3:1:1), m.p.: 123-126°C.
Embodiment 3
[0392] Example 3: Synthesis of N-tert-butoxycarbonyl-imine-diacetyl tert-butylamine
[0393] 13g (56.3mmol) of N-tert-butoxycarbonyl-imine-diacetic acid was dissolved in 180mL of anhydrous tetrahydrofuran, and 24.77mL of N-methylmorpholine was added dropwise at -15°C, followed by 29.3mL (225.2mmol) of chlorine After 3 minutes of tert-butyl formate, 24.77 mL (225.2 mmol) of tert-butylamine was added dropwise. After 3 hours, it was detected by TLC that the raw materials had been reacted. The tetrahydrofuran was spun off, dissolved in ethyl acetate, and the insoluble matter was filtered off. The ester layer was washed with sodium bicarbonate, water, citric acid, and saturated brine, and dried over anhydrous sodium sulfate. Concentrate under reduced pressure, add petroleum ether for crystallization. 13.51 g of a white solid was obtained, with a yield of 70.0%. Rf = 0.54 (chloroform:methanol:acetic acid, 20:1:0.5), m.p.: 139-141°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com