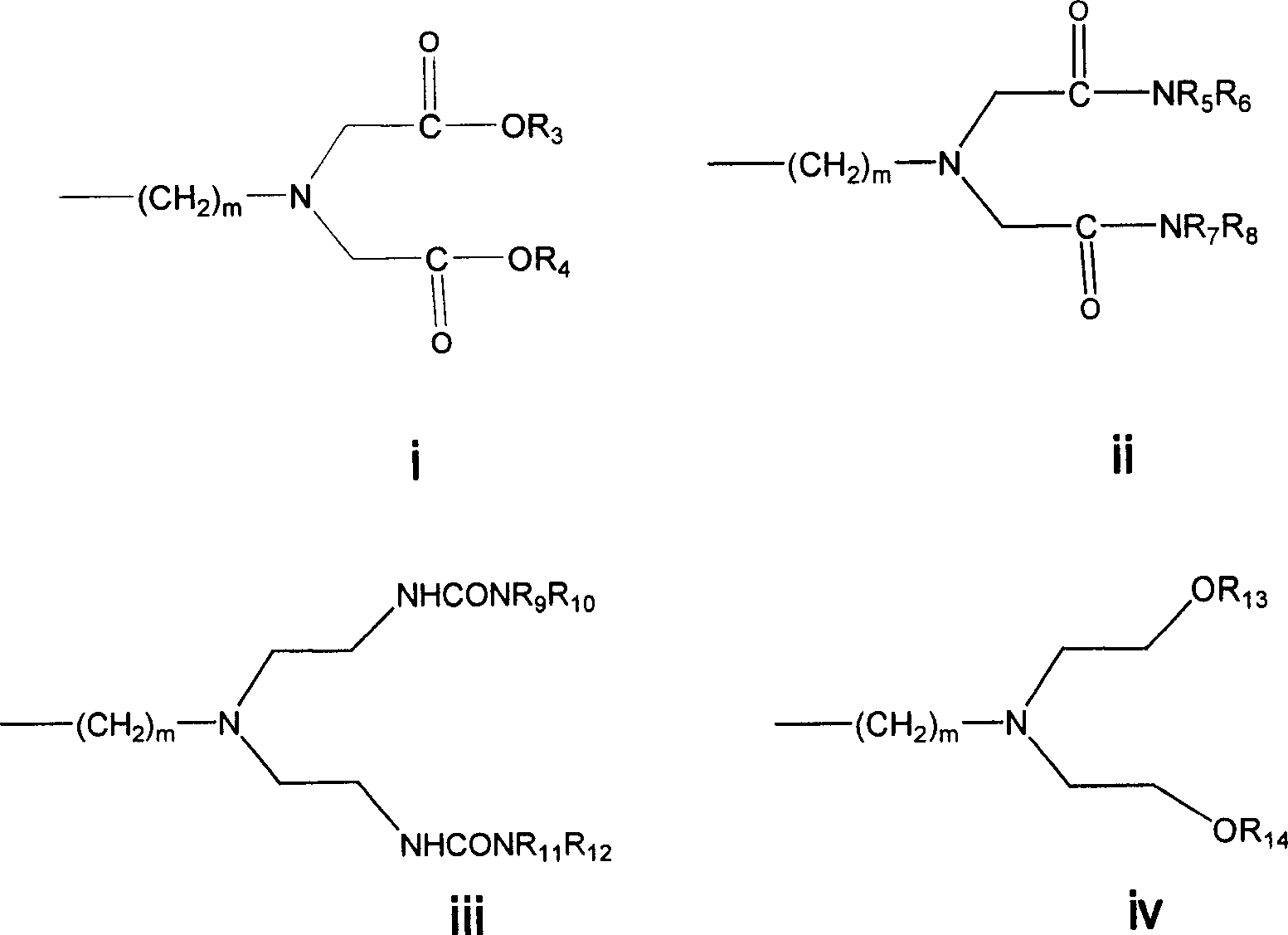
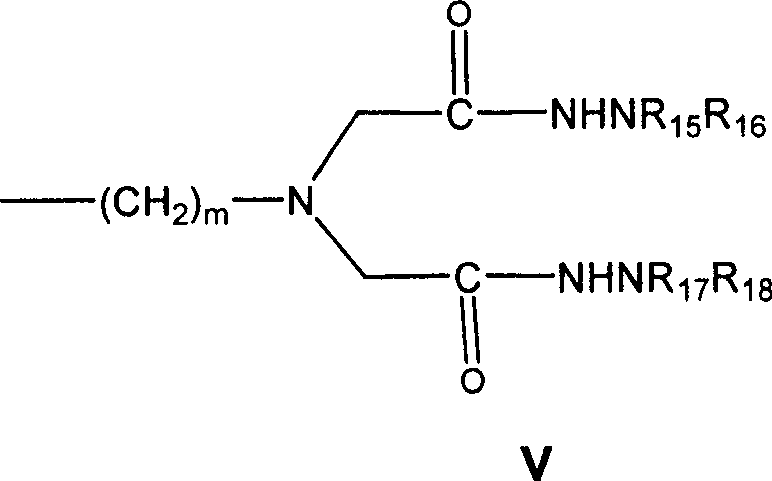
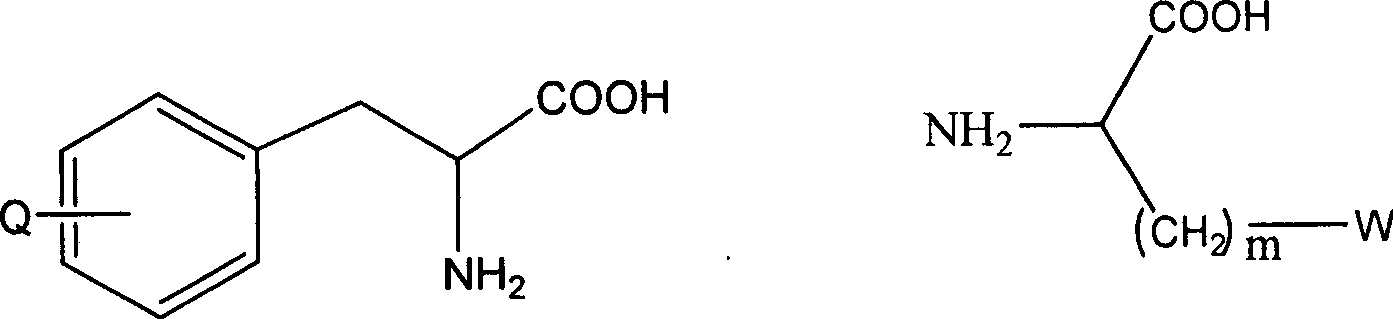LHRH antagonist with low-histamine releasing function
A technology of sex hormones and uses, applied in the field of decapeptide derivatives, to achieve the effect of low histamine release side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0320] Example 1: Boc-p-CH 2 Synthesis of Cl-Phe
[0321] Ac-Phe(p-CH 2 Cl)-Phe-OEt (prepared in this laboratory according to a conventional method, 5g, 17.6mmol) was refluxed in 40mL concentrated hydrochloric acid and 100mL dioxane for 10 hours, and then the hydrochloric acid and dioxane were evaporated to obtain a solid without separation and purification Add 50mL of methanol directly under the ice bath, adjust the pH to 9 with TEA, then add 35.2mmol (3.9mL), and then add 4.6g (Boc) 2 O (21.1 mmol), stirred at room temperature for 12 hours, spin off methanol, adjust the pH of the aqueous solution to acidic, extract with ethyl acetate, wash the ester layer twice with water, and dry over anhydrous sodium sulfate. The ester layer was concentrated, and the oil was recrystallized from ethyl acetate-petroleum ether after column chromatography to obtain 2.7 g of white crystals, with a total yield of 49.1%. TLC detection: chloroform:methanol:HOAc (20:1:0.5), Rf=0.6.
Embodiment 2
[0322] Embodiment 2: Boc-Phe (NA B M) synthesis
[0323] Boc-p-CH 2 Cl-Phe (prepared by conventional methods in our laboratory) (1.75g, 5mmol) and dibenzyl iminodiacetate (1.88g, 6mmol, synthesized according to the method described in the following references: Huang Weide, Chen Changqing, Polypeptide Synthesis, Science Press, 1985, p47) was placed in a 100ml round bottom flask, and 50mL of ethanol was added to dissolve it. After adding TEA (1.69mL, 12mmol) in an ice bath, stir at room temperature for 72 hours, spin off ethanol, adjust the pH of the aqueous solution to alkaline, wash with ether and then adjust the pH of the aqueous phase to acidic, extract with ethyl acetate, wash twice with water, and The layer was dried over anhydrous sodium sulfate. The ester layer was concentrated, and the solid was recrystallized from ethyl acetate-petroleum ether to obtain 1.80 g of white crystals, with a yield of 61%. TLC detection: chloroform:methanol:HOAc (20:1:0.5), Rf=0.7.
Embodiment 3
[0324] Example 3: Boc-p-NH 2 Synthesis of (PhOCO)-Phe
[0325] Boc-p-NH 2 -Phe (1.40g, 5mmol, synthesized according to the method described in the following references: Theobald, P., Porter J., Rivier C., et al, J.Med.Chem., 1991, 34, 2395-2402) placed In a 150mL round bottom flask, add 30mL of water and 9.8mL of 10% sodium carbonate aqueous solution to dissolve them, and then add 30mL of 1,4-dioxane. Phenoxycarbonyl chloride (0.63 mL, 5 mmol) was added under ice cooling. After reacting for 1 hour, 1,4-dioxane was spun off, and citric acid was acidified to acidity. It was extracted with ethyl acetate, and the ester layer was washed with water and saturated brine successively, and dried over anhydrous sodium sulfate. The ester layer was concentrated, 1.3 g of white crystals were obtained by column chromatography, and the yield was 65%. m.p.260°C (decomposition), TLC detection: chloroform:methanol:acetic acid (9:1:0.5), Rf=0.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com