Nimesulide medicine composition for improving dissolution performance and preparation method thereof
A technology of composition and medicine, which is applied in the field of nimesulide pharmaceutical composition and its preparation to improve the dissolution performance, can solve problems such as difficulties in supply, expensive equipment, and increased dissolution of insoluble drugs, so as to improve bioavailability, The effect of great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
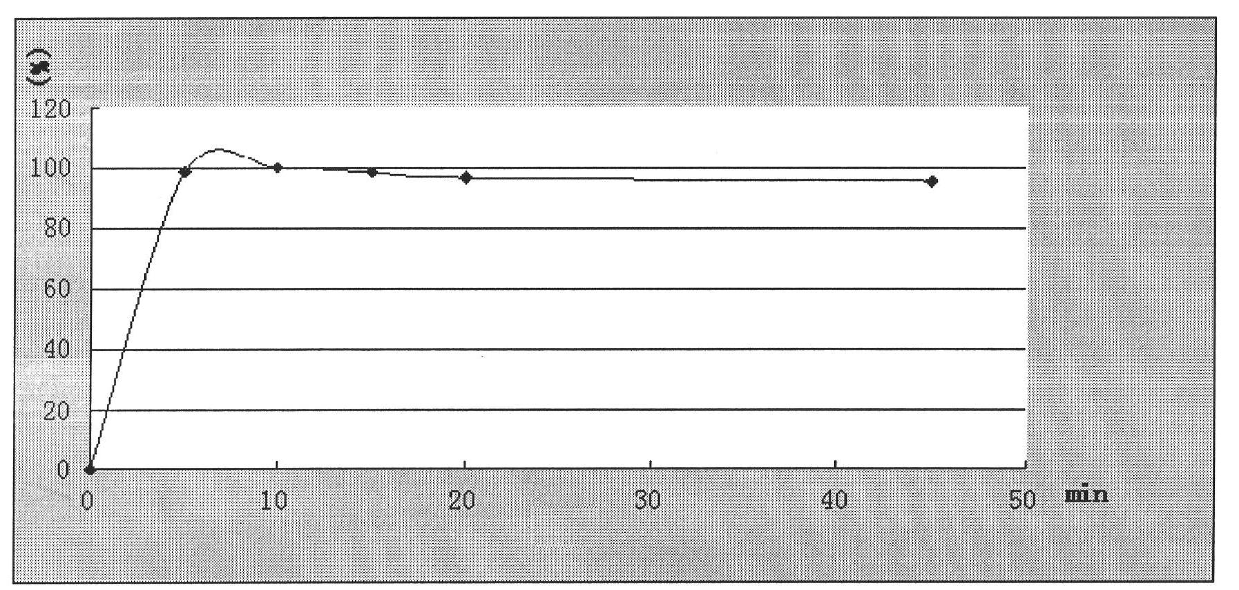
[0058] Embodiment 1: granule (making 1000 bags)
[0059] Ingredients Quantity / bag (g)
[0060] Nimesulide 50
[0061] PEG400 250
[0062] Microcrystalline Cellulose 1000
[0063] Silica 50
[0064] Sodium starch glycolate 71
[0065] Hypromellose 19.5
[0066] 65% ethanol appropriate amount (disappears during processing)
[0067] method:
[0068] (1) Add nimesulide into PEG400, stir and mix;
[0069] (2) Pass microcrystalline cellulose and silicon dioxide through an 80-mesh sieve, and mix well; add the mixture of step 1), and mix well;
[0070] (3) add sodium starch glycolate, mix;
[0071] (4) prepare 2% hypromellose ethanol solution with 60% ethanol as binder;
[0072] (5) wet granulation of the mixture of step 3) with the binder of step 4);
[0073] (6) The granules prepared in step 5) were dried at 65°C.
Embodiment 2
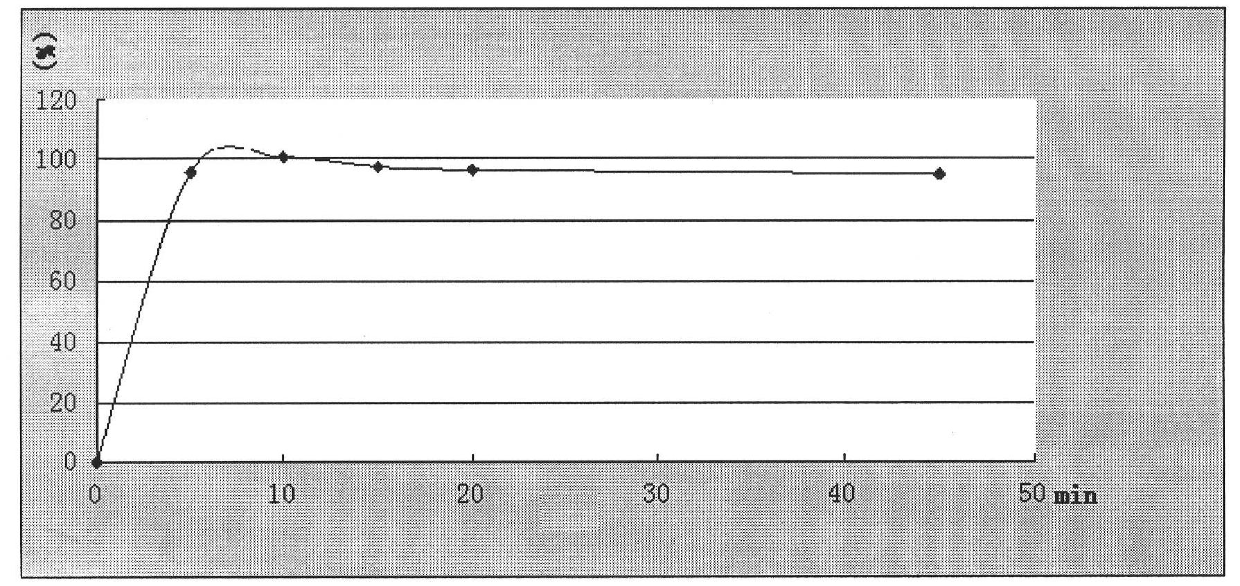
[0074] Embodiment 2: granules (1000 bags)
[0075] Ingredients Quantity / bag (g)
[0076] Nimesulide 50
[0077] PEG300 100
[0078] Microcrystalline Cellulose 500
[0079] Silica 25
[0080] Sodium starch glycolate 40
[0081] Hypromellose 14.5
[0082] 60% ethanol appropriate amount (disappears during processing)
[0083] method:
[0084] 1) Add nimesulide into PEG400, stir and mix;
[0085] 2) Pass the microcrystalline cellulose and silicon dioxide through an 80-mesh sieve, and mix well; add the mixture in step 1), and mix well;
[0086] 3) Add sodium starch glycolate and mix well;
[0087] 4) prepare 2% hypromellose ethanol solution with 60% ethanol as binder;
[0088] 5) wet granulating the mixture of step 3) with the binder of step 4);
[0089] 6) The granules produced in step 5) are dried at 65°C.
Embodiment 3
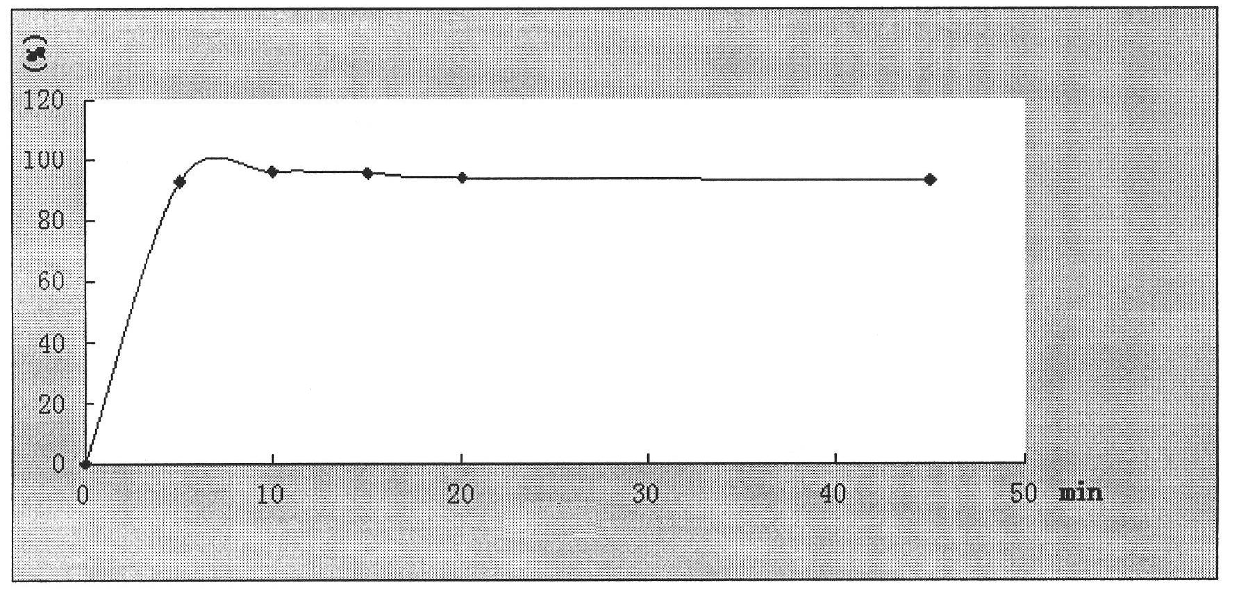
[0090] Embodiment 3: granule (making 1000 bags)
[0091] Ingredients Quantity / bag (g)
[0092] Nimesulide 50
[0093] PEG200 100
[0094] Lactose 1000
[0095] Silica 50
[0096] Sodium starch glycolate 63
[0097] Hypromellose 8.1
[0098] 60% ethanol appropriate amount (disappears during processing)
[0099] method:
[0100] 1) Add nimesulide into PEG400, stir and mix;
[0101] 2) Pass lactose and silicon dioxide through an 80-mesh sieve, and mix well; add the mixture in step 1), and mix well;
[0102] 3) Add sodium starch glycolate and mix well;
[0103] 4) Prepare 2% hypromellose ethanol solution with 60% ethanol as binder;
[0104] 5) wet granulating the mixture of step 3) with the binder of step 4);
[0105] 6) The granules produced in step 5) are dried at 65°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com