Fullerene multi-nitrogen heterocyclic water-soluble derivatives as well as preparation method and application thereof
A fullerene and water-soluble technology, applied in drug delivery, emulsion delivery, organic chemistry, etc., can solve the problems of easy cross-interference, large steric hindrance effect, unfavorable coupling reaction, etc., to achieve rich variety and improve Solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
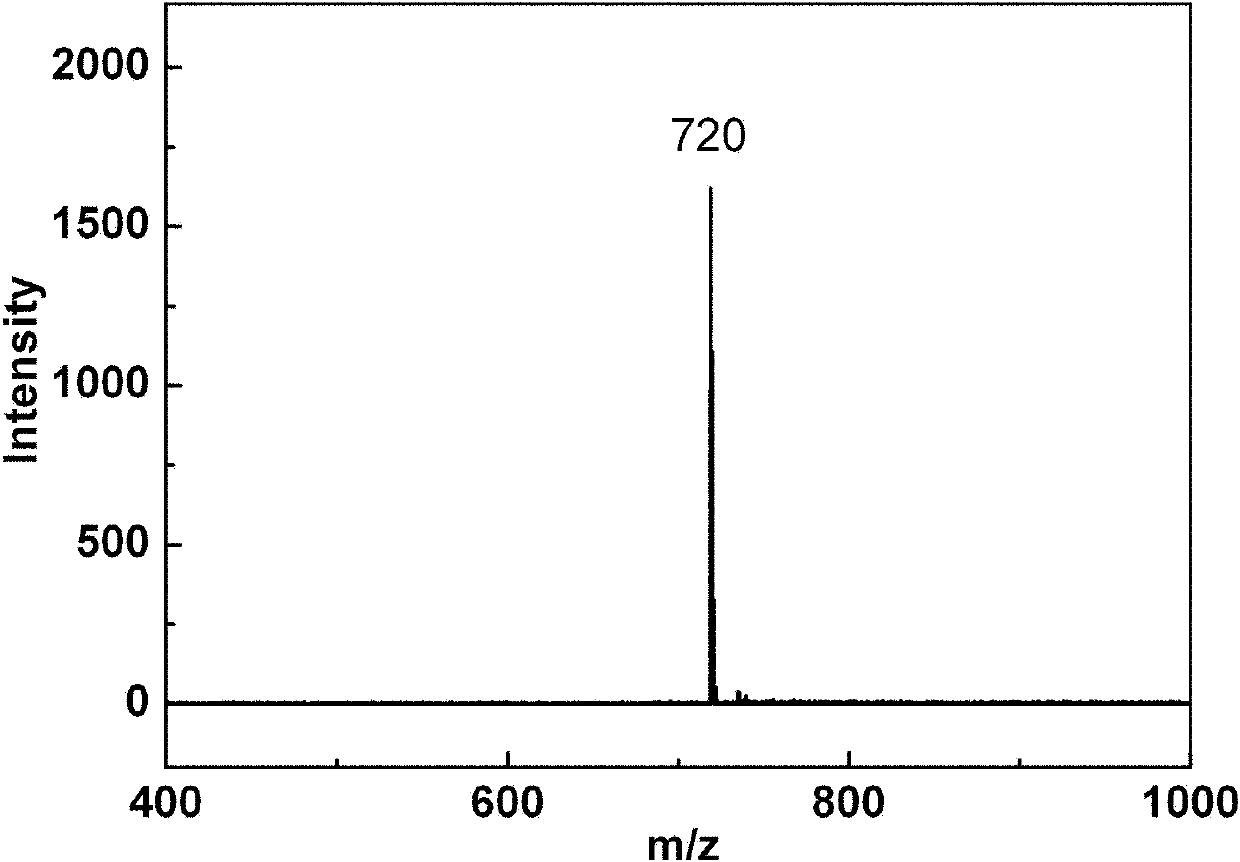
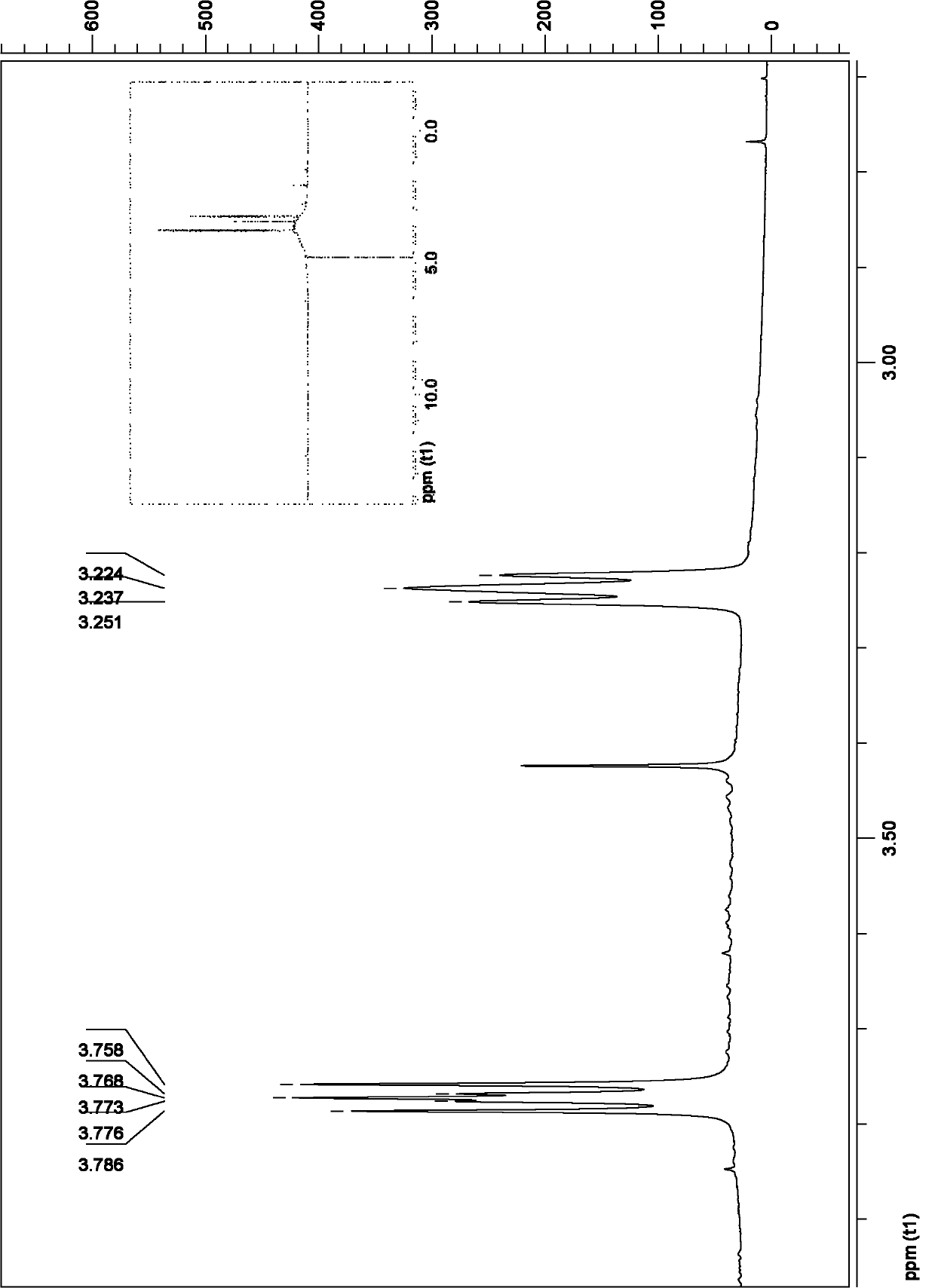
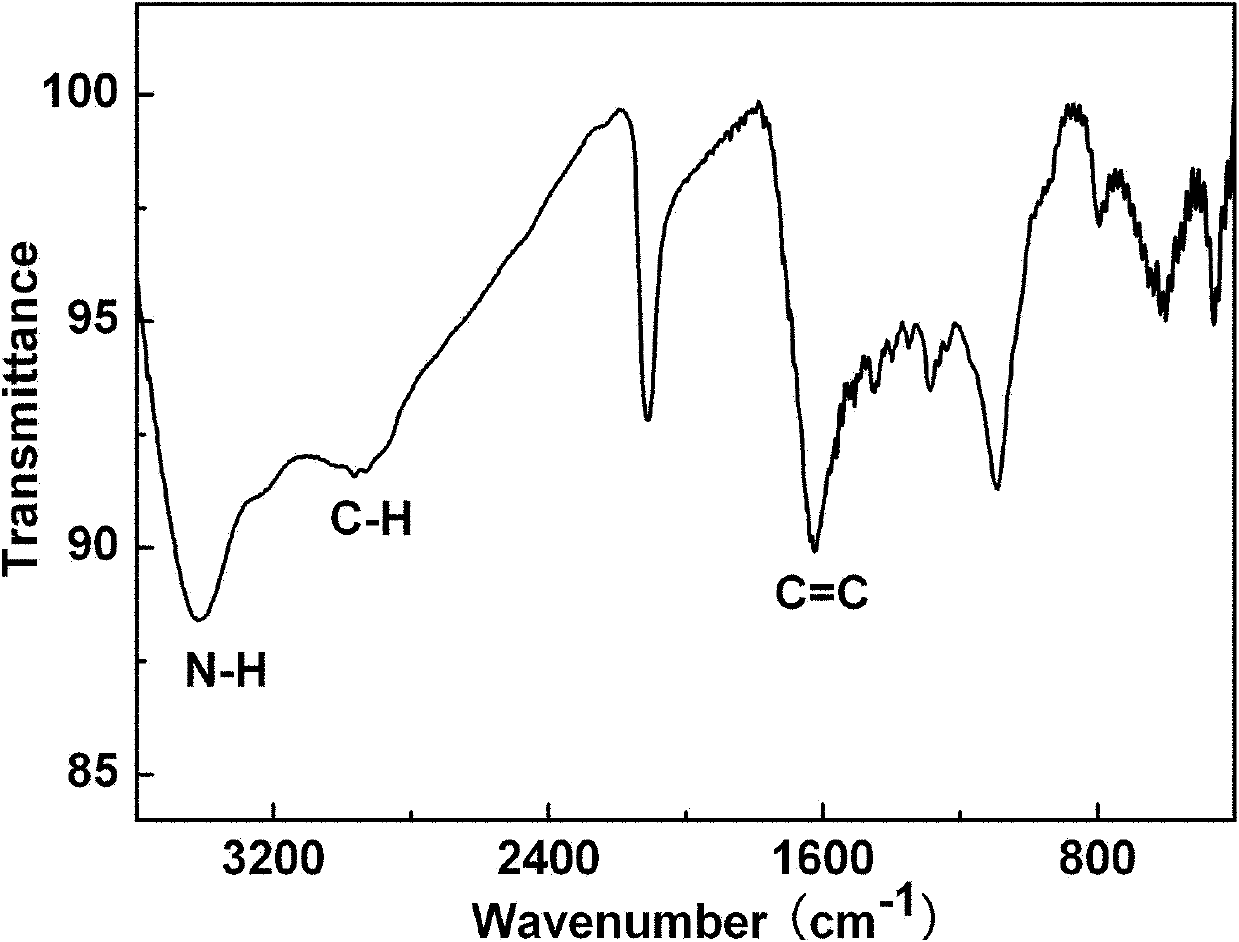
[0044] Example 1: C 60 Multiple addition product C with 2-azidoethylamine 60 (NCH 2 CH 2 NH 2 ) x (Compound 1) Preparation
[0045] Weigh 0.10mmol of C 60 Dissolve in 15ml of chlorobenzene, under inert gas protection at normal temperature, stir with a magnet, slowly add 2-azidoethylamine solution (5.0mmol of 2-azidoethylamine dissolved in 5ml of chlorobenzene) dropwise within 30 minutes, After the dropwise addition was completed, the temperature was slowly raised to 80° C., and the reaction was heated for 3 days, and the temperature was raised to reflux for 1 day, and the reaction was stopped. During heating, bright purple C 60 The solution gradually turned orange-red and continued to darken to brown. After the reaction liquid was cooled to room temperature, the solvent was distilled off under reduced pressure, and the residue was washed with toluene, diethyl ether, and methanol successively to obtain a brownish black solid, namely compound 1, with a yield of 58%.
[...
Embodiment 2
[0053] Example 2: C 60 Multiple addition product C with 2-azidoacetic acid 60 (NCH 2 COOH) x (Compound 2) and C 60 (NCH 2 COONa) x (Compound 3) Preparation
[0054] Weigh 0.10mmol of C 60 Dissolve in 15ml of chlorobenzene, under the protection of inert gas at normal temperature, stir with a magnet, slowly add 2-azidoacetic acid solution (2.0mmol of 2-azidoacetic acid dissolved in 5ml of chlorobenzene) dropwise within 30 minutes, dropwise After completion, the temperature was slowly raised to 50° C., the reaction was heated for 24 hours, and the temperature was continuously raised to reflux for 5 hours. The solvent was removed, and the residue was washed successively with toluene, diethyl ether, and methanol to obtain a tan solid, namely compound 2, with a yield of 95%.
[0055] Weigh 10mg of compound 2 solid powder, dissolve it in an appropriate amount of 1mol / L NaOH, mix well, control the pH value at 12-13, and separate with Sephadex G-15 chromatographic column, neutr...
Embodiment 3
[0058] Example 3: C 60 Multiple addition product C with 3-azidopropionic acid 60 (NCH 2 CH 2 COOH) x (Compound 4) and C 60 (NCH 2 CH 2 COONa) x (Compound 5) Preparation
[0059] Weigh 0.10mmol of C 60 Dissolve in 15ml of chlorobenzene, under the protection of inert gas at normal temperature, stir with a magnet, slowly add 3-azidopropionic acid solution (2.0mmol 3-azidopropionic acid dissolved in 5ml of chlorobenzene) dropwise within 30 minutes, drop After the addition was completed, the temperature was raised slowly to 60° C., and the reaction was heated for 24 hours, and the temperature was continued to rise and reflux for 10 hours. The solvent was removed, and the residue was washed successively with toluene and ethyl acetate to obtain a brownish-yellow solid, namely compound 4, with a yield of 92%.
[0060] The preparation method of Compound 5 was the same as that of Compound 3 in Example 2, except that Compound 2 was replaced by Compound 4 to obtain purified Comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com