Method for synthesizing ibuprofen and analogues thereof
A technology of compounds and substances, applied in the field of compound synthesis, can solve problems such as insufficient safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
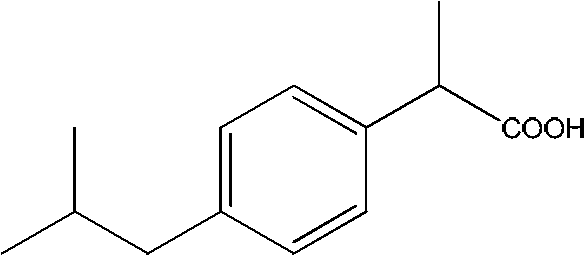
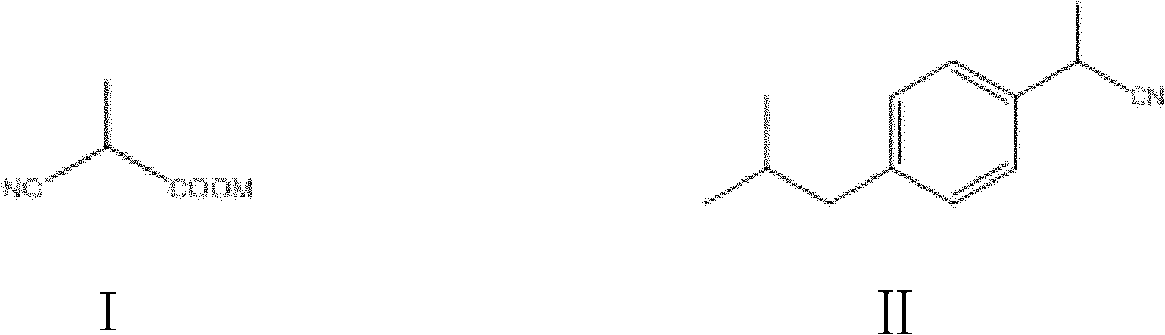
[0160](1) Preparation of 2-(4-isobutylphenyl) propionitrile
[0161] In an oven-dried 100 mL Schlenk flask equipped with a magnet, add dipolyallylpalladium chloride (0.0146 g, 0.040 mmol), 9,9-dimethyl-4,5-bis(di tert-butylphosphino)xanthene (0.0693 g, 0.120 mmol), 1-bromo-4-isobutylbenzene (8.52 g, 40 mmol) and potassium 2-cyanopropionate (6.58 g, 48 mmol). The lid is coated with a vacuum grease grinding plug, connected to the Schrank vacuum line, the air in the container is drained and filled with nitrogen, repeat 3 times, and 40mL of mesitylene is added under the reverse flow of nitrogen. After the addition, cover with a stopper, put it into a 140°C oil pan after clamping, and stir for 20 hours. After the reaction was completed, it was directly separated by chromatographic column to obtain a light yellow liquid with a yield of 96%.
[0162] (2) Preparation of 2-(4-isobutylphenyl) propionic acid
[0163] Add 2-(4-isobutylphenyl)propionitrile (5.62g, 30mmol) and sodium hyd...
Embodiment 2
[0165] (1) Preparation of 2-(4-isobutylphenyl) acetonitrile
[0166] In an oven-dried 100 mL Schlenk bottle equipped with a magnet, add dipolyallylpalladium chloride (0.0146 g, 0.040 mmol), 2-bicyclohexylphosphine-2′,6′-dimethoxy phenylbiphenyl (0.0493g, 0.120mmol), 1-chloro-4-isobutylbenzene (6.75g, 40mmol) and sodium cyanoacetate (5.14g, 48mmol). The lid is coated with a vacuum grease grinding plug, connected to the Schrank vacuum line, the air in the container is drained and filled with nitrogen, repeat 3 times, and 40mL of mesitylene is added under the reverse flow of nitrogen. After the addition, cover with a stopper, put it into a 140°C oil pan after clamping, and stir for 20 hours. After the reaction was completed, it was directly separated by chromatographic column to obtain a light yellow liquid with a yield of 97%.
[0167] (2) Preparation of 2-(4-isobutylphenyl) propionitrile
[0168] In a 150ml round bottom flask equipped with a magnet was charged 2-(4-isobutylp...
Embodiment 3
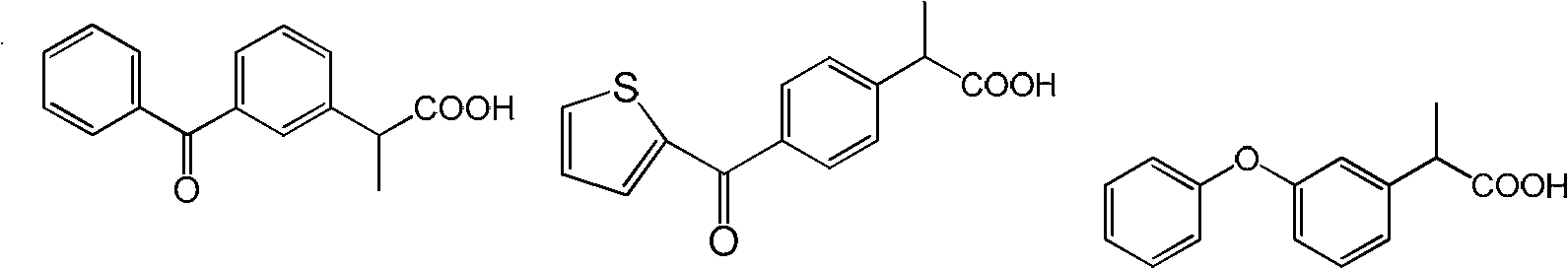
[0173] (1) Preparation of 2-(3-benzoylphenyl) propionitrile
[0174] In an oven-dried 100 mL Schlenk flask equipped with a magnet, add dipolyallylpalladium chloride (0.0146 g, 0.040 mmol), 9,9-dimethyl-4,5-bis(di tert-butylphosphino)xanthene (0.0693 g, 0.120 mmol), 3-bromophenylbenzophenone (10.44 g, 40 mmol) and potassium 2-cyanopropionate (6.58 g, 48 mmol). The lid is coated with a vacuum grease grinding plug, connected to the Schrank vacuum line, the air in the container is drained and filled with nitrogen, repeat 3 times, and 40mL of mesitylene is added under the reverse flow of nitrogen. After the addition, cover with a stopper, put it into a 140°C oil pan after clamping, and stir for 20 hours. After the reaction was completed, it was directly separated by chromatographic column to obtain a light yellow liquid with a yield of 91%.
[0175] (2) Preparation of 2-(3-benzoylphenyl) propionic acid
[0176] Add 2-(3-benzoylphenyl)propionitrile (7.06g, 30mmol) and sodium hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com