Method for preparing single-layer high-activity titanium dioxide thin film
A titanium dioxide, high-activity technology, applied in the direction of metal material coating process, etc., can solve the problem that the photocatalytic efficiency of titanium dioxide cannot be satisfied, titanium dioxide powder is difficult to make a dense and strong film, and the application is limited, so as to achieve a controllable film thickness , enhance the transfer efficiency, combined with a firm effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Anhydrous ethanol and deionized water were ultrasonically cleaned 0.25mm×10mm×30mm titanium flakes, and after vacuum drying at 80°C, clean titanium flakes with no oxide layer on the surface were obtained. Then, the clean titanium sheet was placed in an 80 mL stainless steel reactor containing 40 mL of 10 mM diluted hydrofluoric acid aqueous solution and lined with polytetrafluoroethylene. After the reaction kettle was sealed, it was put into an oven and heated at 140°C for 10 hours. The reacted titanium flakes were taken out, washed with deionized water and dried at 80°C to obtain a titanium flake with a single-layer (001) crystal plane titanium dioxide film formed on the surface .
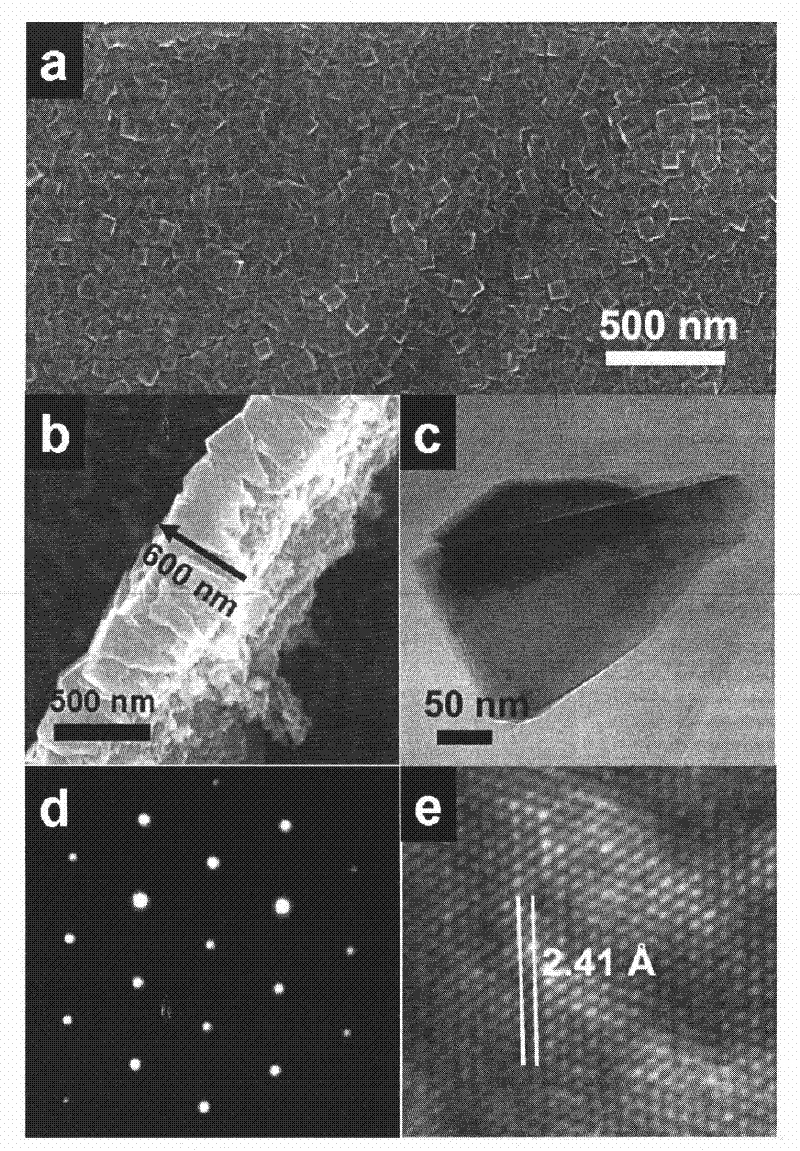
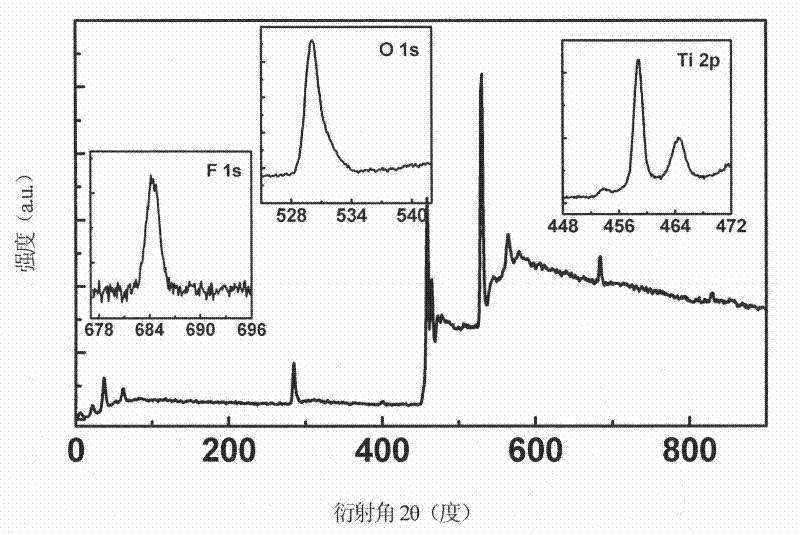
[0029] Such as figure 1 As shown, the exposed surface of the titanium dioxide film formed on the titanium sheet is a highly active (001) crystal plane. The film is composed of tooth-like titanium dioxide crystal particles with a particle size of 130 nm and high crystallinity of the film. The ...
Embodiment 2
[0035] Anhydrous ethanol and deionized water were ultrasonically cleaned 0.25mm×10mm×30mm titanium flakes, and after vacuum drying at 80°C, clean titanium flakes with no oxide layer on the surface were obtained. Then, the clean titanium sheet was placed in an 80 mL stainless steel reactor containing 40 mL of 8 mM dilute hydrofluoric acid aqueous solution and lined with polytetrafluoroethylene. After the reactor was sealed, it was put into an oven and heated at 120°C for 10 hours. The reacted titanium flakes were taken out, washed with deionized water and dried at 80°C to obtain a titanium flake with a monolayer (001) crystal plane titanium dioxide film formed on the surface .
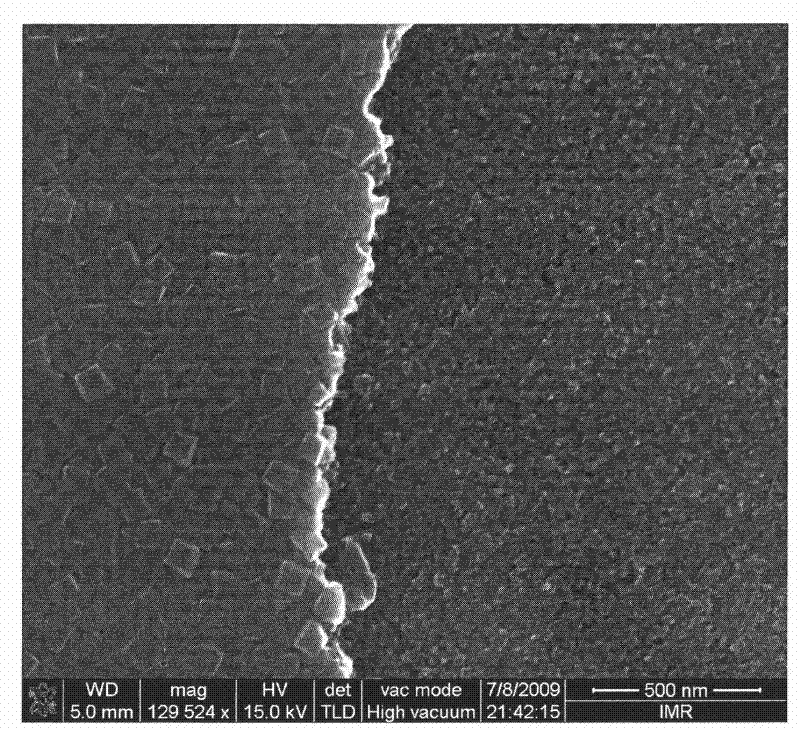
[0036] Such as Image 6 As shown, the titanium dioxide (001) crystal face film synthesized under this condition is a titanium dioxide crystal, the size of the titanium dioxide crystal particle is 300nm, the film is composed of anatase and rutile phase titanium dioxide, the ratio of anatase and rutile It i...
Embodiment 3
[0038] Anhydrous ethanol and deionized water were ultrasonically cleaned 0.2 g of titanium blocks, and after vacuum drying treatment at 80° C., a clean titanium block with no oxide layer on the surface was obtained. Then, the clean titanium block was placed in an 80 mL stainless steel reactor containing 40 mL of 15 mM dilute hydrofluoric acid aqueous solution and lined with polytetrafluoroethylene. After the reaction kettle was sealed, it was put into an oven and heated at 140℃ for 15h. The reacted titanium block was taken out, washed with deionized water and dried at 100℃ to obtain a titanium block with a monolayer (001) crystal plane titanium dioxide film formed on the surface .
[0039] Such as Figure 7 As shown, the titanium dioxide (001) crystal face film synthesized under this condition is a titanium dioxide crystal, the size of the titanium dioxide crystal particle is 120nm, the film is composed of anatase and rutile phase titanium dioxide, the ratio of anatase and rutil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com