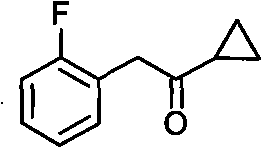
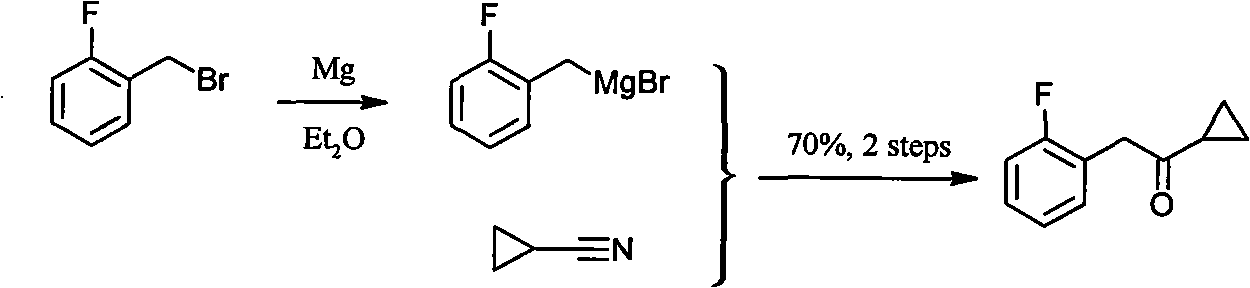
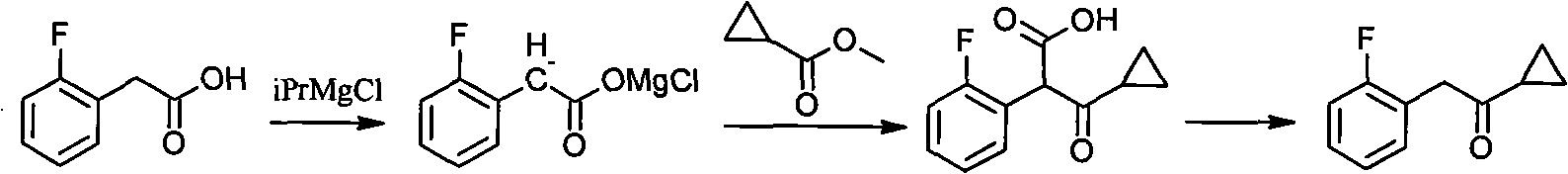
Method for preparing cyclopropyl o-fluobenzyl ketone
A technology of o-fluorobenzyl ketone and cyclopropyl, applied in the direction of condensation to prepare carbonyl compounds, organic chemistry, etc., to achieve the effect of good safety and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Add 15.7g zinc powder (1.6eq.), anhydrous LiCl 9.5g (1.5eq.) into a 500ml dry four-necked reaction flask, add 50ml tetrahydrofuran, 1.4g dibromoethane (5% mol), Heated to reflux for 10 min under nitrogen protection. After cooling slightly, 1.5 ml (1% mol) of 1.0 M TMS-Cl in tetrahydrofuran was added, and heated to reflux again for 10 min. Cool to 20°C, start dropwise adding 21.7g o-fluorobenzyl chloride in 250ml tetrahydrofuran solution, during the dropwise addition, use ice water to control the reaction temperature not to exceed 25°C, dropwise is completed in about 2 hours, continue to keep stirring for 1.5 hours, and filter to obtain 0.5 M O-fluorochlorobenzyl zinc reagent. The yield is nearly quantitative.
[0041] (2) Under the protection of nitrogen, 1.0g of anhydrous CuCl 2 (5% mol) and 0.64 g of anhydrous LiCl (10% mol) were added to a 500 ml four-necked reaction flask, 75 ml of tetrahydrofuran was added, and stirred for 30 min to obtain a lithium tetrachl...
Embodiment 2
[0043] (1) Add 15.7g zinc powder (1.6eq.), anhydrous LiCl 9.5g (1.5eq.) into a 500ml dry four-necked reaction flask, add 50ml tetrahydrofuran, 1.4g dibromoethane (5% mol), Heated to reflux for 10 min under nitrogen protection. After cooling slightly, 1.5 ml (1% mol) of 1.0 M TMS-Cl in tetrahydrofuran was added, and heated to reflux again for 10 min. Cool to 20°C, start dropwise adding 21.7g o-fluorobenzyl chloride in 250ml tetrahydrofuran solution, during the dropwise addition, use ice water to control the reaction temperature not to exceed 25°C, dropwise is completed in about 2 hours, continue to keep stirring for 1.5 hours, and filter to obtain 0.5 M O-fluorochlorobenzyl zinc reagent. The yield is nearly quantitative.
[0044] (2) First prepare 21.7 g of zinc reagent solution of o-fluorobenzyl chloride according to experiment (1), add 23.5 g (1.5 eq.) of cyclopropylformyl chloride to this solution, and heat to reflux overnight under nitrogen protection. Cool to room tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com