Methods for preparing fluoxymesterone and intermediate thereof
A technique for an intermediate and fluoxymethylene, which is applied in the field of preparation of steroid compounds, can solve the problems of insufficient fluoxymester process route, difficulty in obtaining starting materials, production waste, etc., and achieves reduction of production cost and industrialization conditions, and easy operation. , the effect of the line is reasonable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
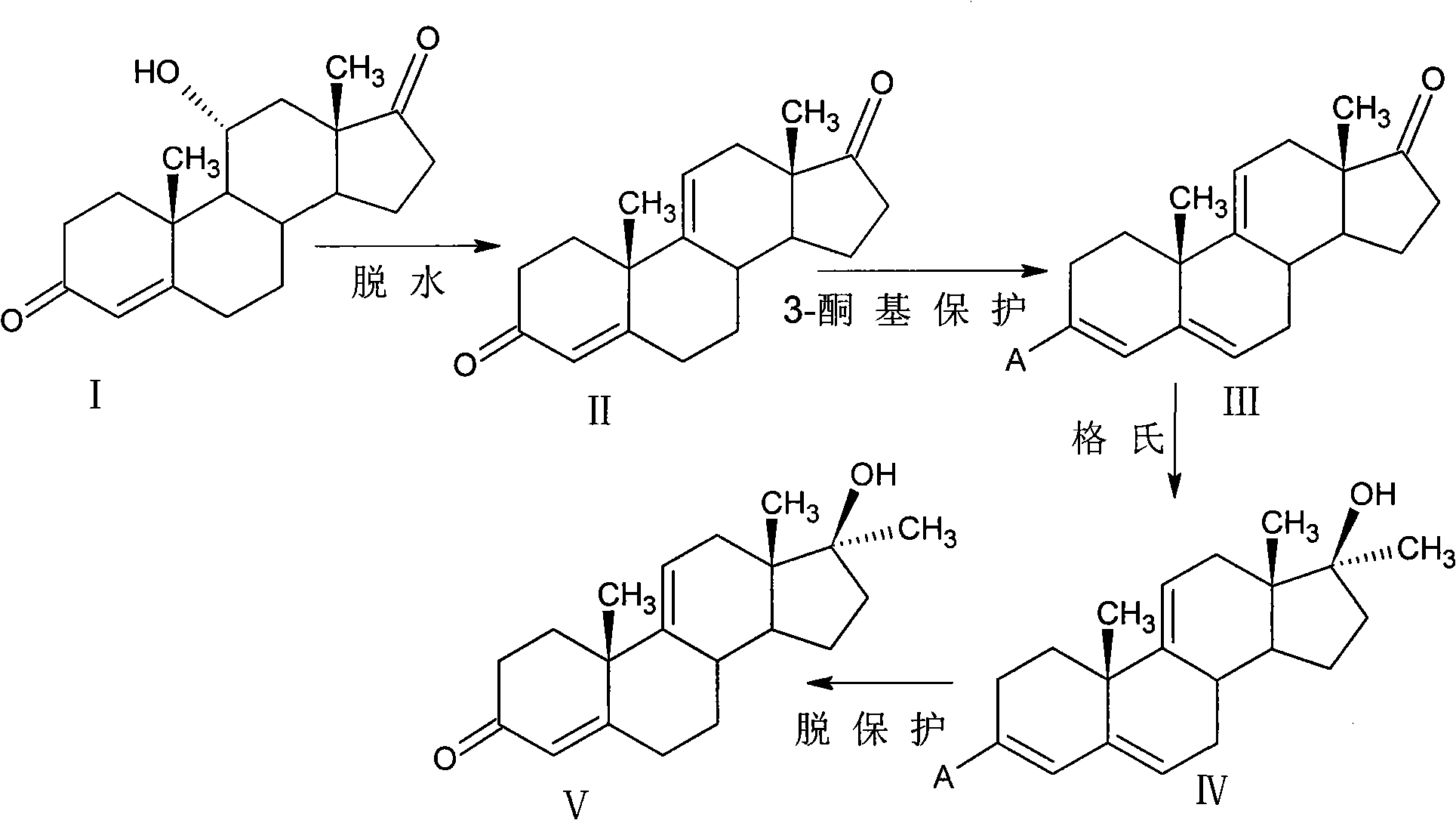
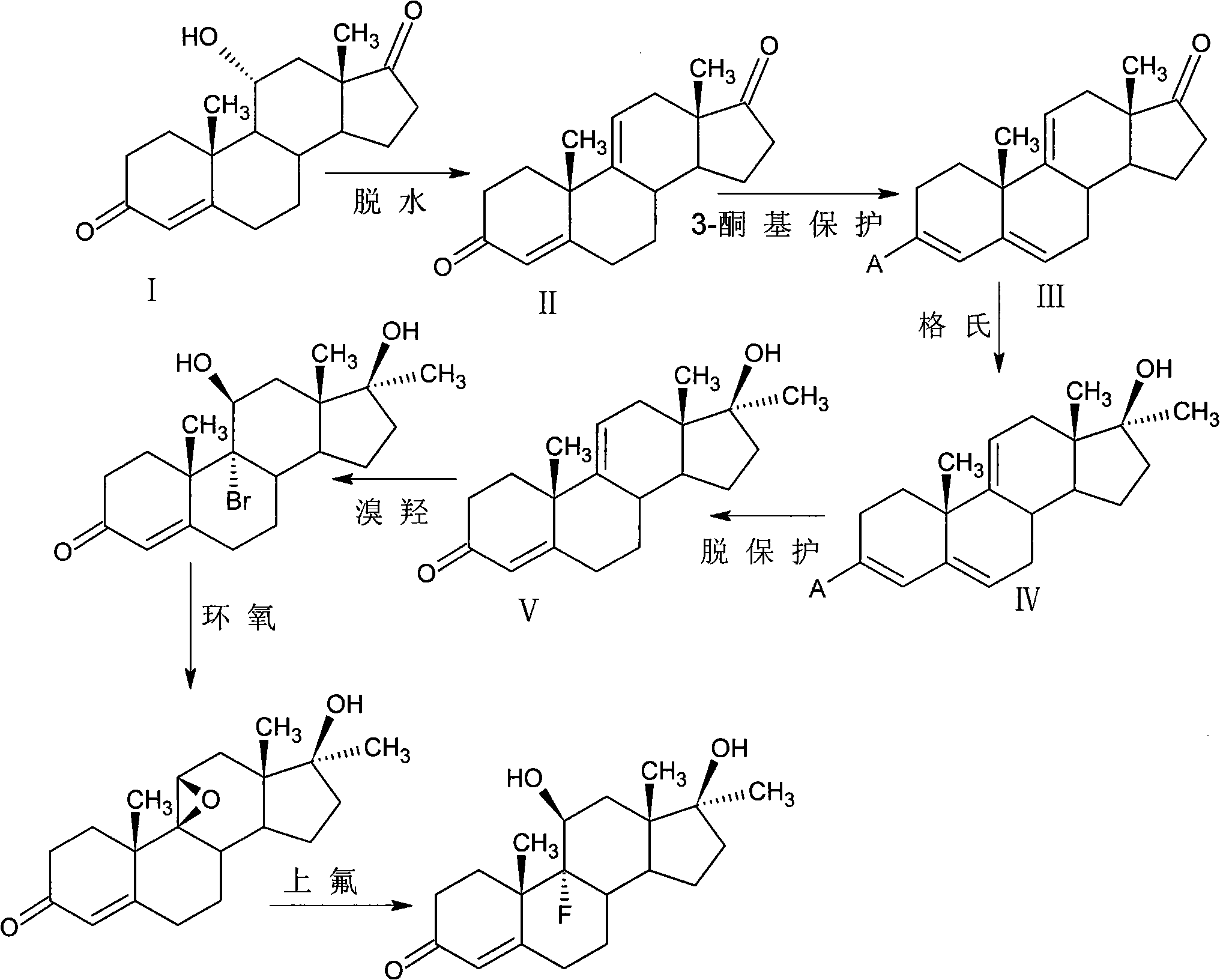
[0031] Preparation of 9,11-double bond-17α-methyltestosterone
[0032]
[0033] (1) 9, 11 dehydration
[0034] Put 10g11αhydroxy-4AD and 45ml tetrahydrofuran into the reaction flask under stirring, cool down to -60°C, then add 9gPCl 5 Add it to the reaction flask, control the temperature to react at -50~-60℃ for 2 hours, monitor with thin layer chromatography until the reaction ends without raw materials, pour the reaction solution into 50 times of ice water to dilute, add 10g of flake alkali to neutralize, and precipitate out crystals. It was left to stand, filtered, the filter cake was washed with water until neutral, and dried to obtain 9.2 g of compound 4,9(11)-androstadiene-3,17-dione (II).
[0035] (2) 3-position keto group protection
[0036] 9.2g of 4,9(11)-androstadiene-3,17-dione (II) and 200ml of benzene obtained in step (1) were put into a reaction flask under stirring, and 12ml of pyrrolidine and 10mg of p-toluenesulfonic acid were added. acid, heated to ref...
Embodiment 2
[0043] Preparation of 9,11-double bond-17α-methyltestosterone II
[0044]
[0045] (1) 9, 11 dehydration
[0046] Put 10g11αhydroxy-4AD and 45ml dioxane into the reaction flask under stirring, cool down to -50℃, then add 9.5gPOCl 3 Add it into the reaction flask, control the temperature to react at -50~-60℃ for 3 hours, monitor with thin layer chromatography until the reaction ends without raw materials, pour the reaction solution into 50 times ice water to dilute, add 10g of flake alkali to neutralize, and precipitate out crystals. Set aside, filter, wash the filter cake with water until neutral, and dry to obtain 9.1 g of compound 4,9(11)-androstadiene-3,17-dione (II).
[0047] (2) 3-position keto group protection
[0048] 9.1g of 4,9(11)-androstadiene-3,17-dione (II) and 200ml of toluene obtained in step (1) were put into a reaction flask under stirring, and 15ml of morpholine and 20mg of p-toluenesulfonic acid were added. acid, heated to reflux for 3h, the reactor wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com