Human embryo retina transformed cell line for producing biological products
A technology of retinal cells and transformed cells, which is applied in the field of biological product preparation and can solve the safety hazards of adenovirus vectors and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
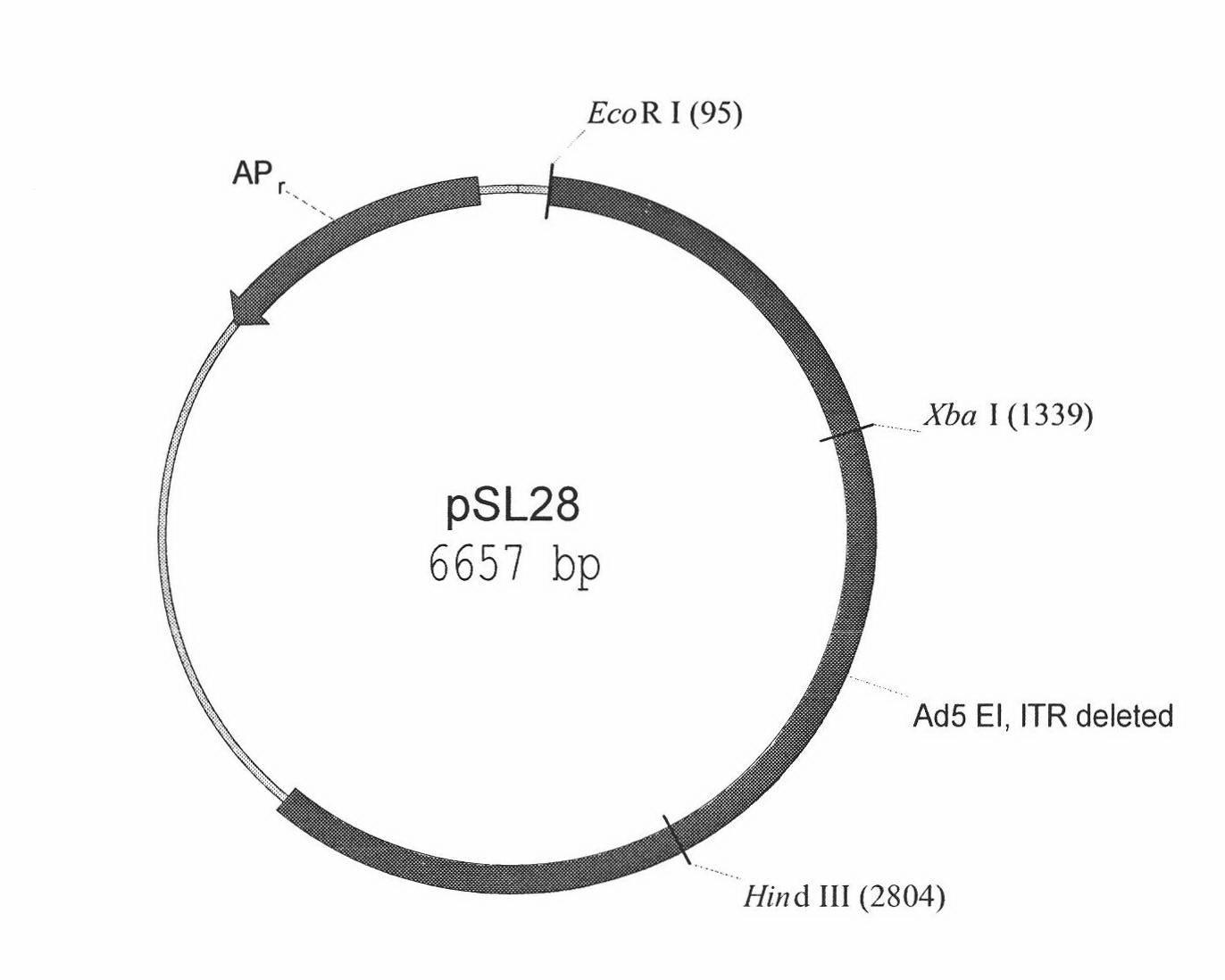
[0010] Construction of embodiment 1pSL28 plasmid
[0011] The pXC1 plasmid contains the human adenovirus gene sequence from bp22 to bp5790 (McKinnon et. al. Gene. 1982, 19, 33-42). We first deleted the Bsa BI fragment in the pXC1 plasmid to obtain the plasmid pSL26. Use the PCR method to amplify the fragment from bp100 to xbaI (bp1339) on the left side of the adenovirus, and the amplification primers are:
[0012] Sense strand primer: 5’-AGA ATT CCC CTT CCA GCT CTC TGC CCC-3’
[0013] Antisense strand primer: 5’-ACA GCT ATC CGT ACT ACT AT-3’
[0014] The amplified fragment was cloned into the T vector pCST, thereby obtaining the plasmid pSL27.
[0015] The EcoRI-XbaI fragment in the pSL27 plasmid was replaced with the EcoRI-XbaI fragment in the pXC1 plasmid to obtain plasmid pSL28. (pSL28 plasmid structure Atlas See attached picture 1)
Embodiment 2
[0016] Example 2 Transformation of Human Embryo Retinal Cells Using the pSL28 Plasmid
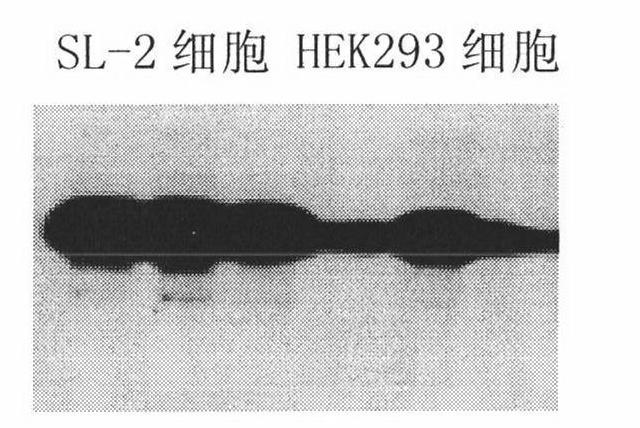
[0017] The pSL28 plasmid was prepared, and in the case of adding the transformation reagent Lipofectmine from Invitrogen Company, the pSL28 plasmid was used to transform human embryonic retinal cells to obtain human embryonic retinal transformed cell lines. We obtained a total of 9 transformed cell clones. After detecting the E1a gene expression of each cell clone, we selected a cell clone that highly expressed the E1a gene and named it SL-2 cell line. Compared with HEK293 cells, SL-2 cells can express E1a gene at a higher level. The test results are shown in Figure 2. It can be seen from the figure that in the experiments repeated three times in parallel, the expression level of E1a gene in SL-2 cells was higher than that in HEK293 cells. SL-2 cells are transformed human embryonic retinal cell lines, which have been preserved in the China Center for Type Culture Collection (Address: Wuha...
Embodiment 3
[0018] Example 3 SL-2 cell line production adenovirus characteristic detection
[0019] We conducted a series of tests on the characteristics of the virus produced by the SL-2 cell line. In the test of cell line stability, we continuously passaged the SL-2 cell line for 40 times, and all subsequent generations of cells could stably express the E1a gene product. Ad5LacZ adenoviral vector (deleted E1 region gene) was used to infect SL-2 cells of various generations, and Ad5LacZ adenoviral vector could grow smoothly in all generations of SL-2 cells. , after being infected with Ad5LacZ adenovirus vector, the progeny virus can be harvested, and each cell can produce 11000--15000 virus particles on average. After counting the number of cells in unit culture area and comparing with HEK293 cells, it was found that in unit culture area, the number of SL-2 cells was 2.5 times that of HEK293 cells. Comparing the total number of viruses produced per unit culture area, it was found that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com