Application of 4-(cyclohexyl)-aminoquinazoline compounds
A compound and drug technology, applied in the field of medical immunology, can solve problems such as body damage, achieve significant effects, avoid immune response, and inhibit immune response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
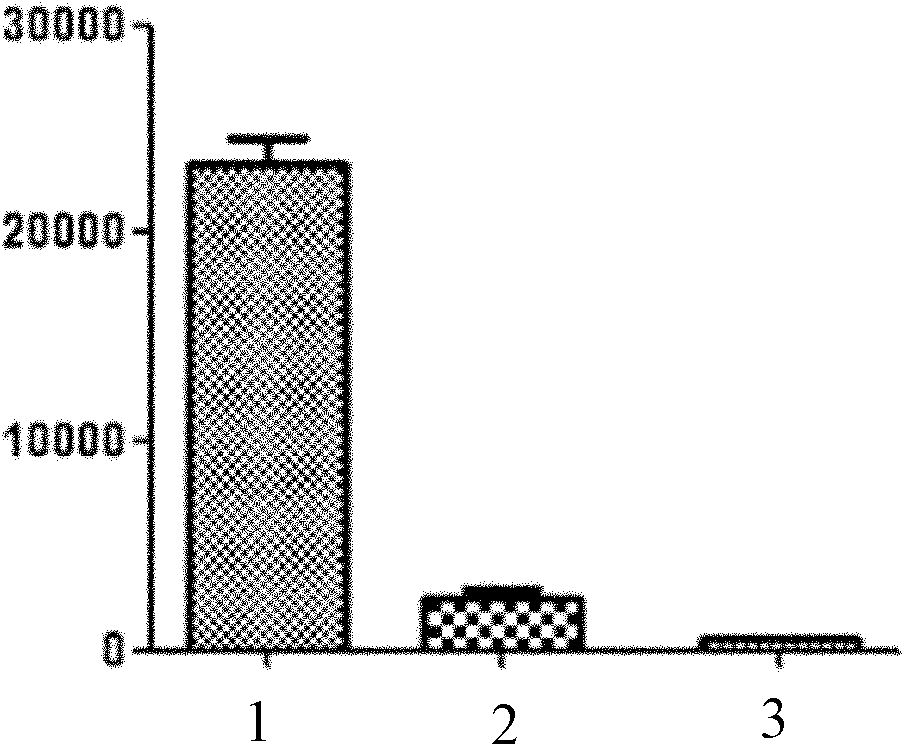
[0040] Example 1: Mouse T cell proliferation inhibition test
[0041] After aseptically separating spleen cells from mice and lysing red blood cells, add them to 96-well culture plates, 1-2×10 5 Cells / 100 μL / well. Add anti-CD3 (final concentration 1 μg / mL), anti-CD28 monoclonal antibody (final concentration 1 μg / mL), recombinant IL-2 (final concentration 10 Unit / mL) to stimulate in vitro, 100 μL / well, suppose adding 4-(cyclohexyl)- Aminoquinazoline was the test group, 3 wells / group; no 4-(cyclohexyl)-aminoquinazoline was used as the control group, 3 wells / group. At the same time, set the group without the above in vitro stimulation and without adding 4-(cyclohexyl)-aminoquinazoline as the blank group, 3 wells / group.
[0042] Three groups of samples were placed at 37°C, 5% CO 2 Incubate under the condition for 66h, add 0.5-1μci3H-TdR 50μL to each well, and continue to cultivate for 12h. Use DYQ-II type multi-head cell harvester to collect samples of each group on 9999 type ...
Embodiment 2
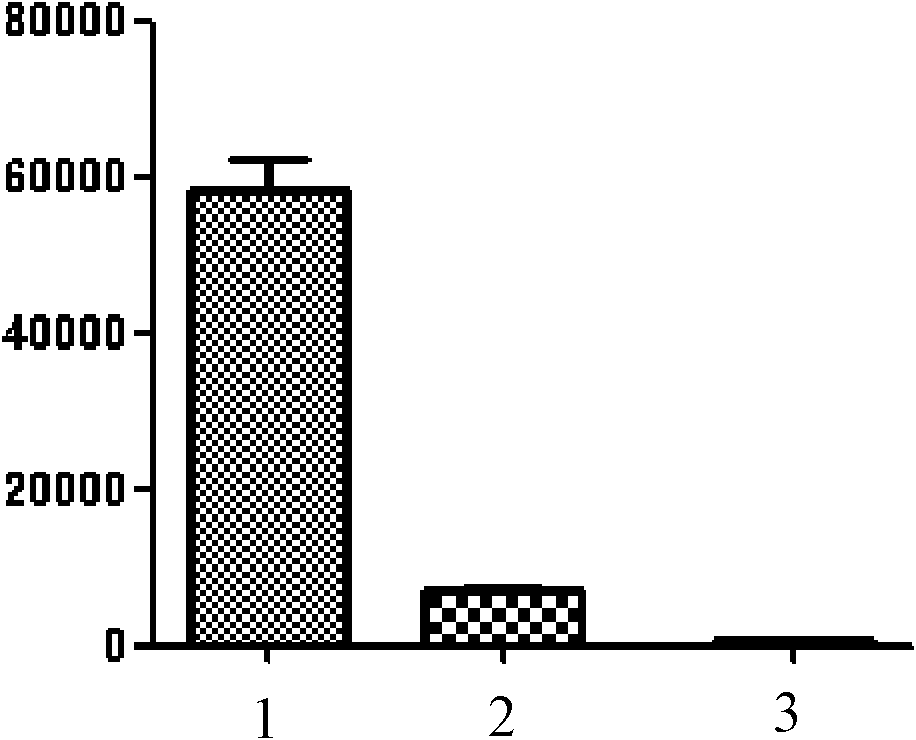
[0044] Embodiment 2: human T cell proliferation inhibition test
[0045] Under sterile conditions, isolate peripheral blood mononuclear cells (PBMC) from heparin anticoagulated blood, and adjust the number of cells to 1-2×10 with 10% FCS RPMI1640 after washing 6 / mL, added to 96-well culture plate, 1-2×10 5 Cells / 100 μL / well. Add T cell mitogen PHA, 100 μL / well, set 4-(cyclohexyl)-aminoquinazoline as the test group, 3 wells / group; no 4-(cyclohexyl)-aminoquinazoline is the control group, 3 wells / group. At the same time, set the group without the above in vitro stimulation and without adding 4-(cyclohexyl)-aminoquinazoline as the blank group, 3 wells / group.
[0046] Three groups of samples were placed at 37°C, 5% CO 2 Incubate for 66h under the condition, add 0.5-1μci to each well 3 H-TdR 50μL, continue to culture for 12h. Use DYQ-II type multi-head cell harvester to collect samples of each group on 9999 type glass fiber filter paper, and count them with β liquid scintilla...
Embodiment 3
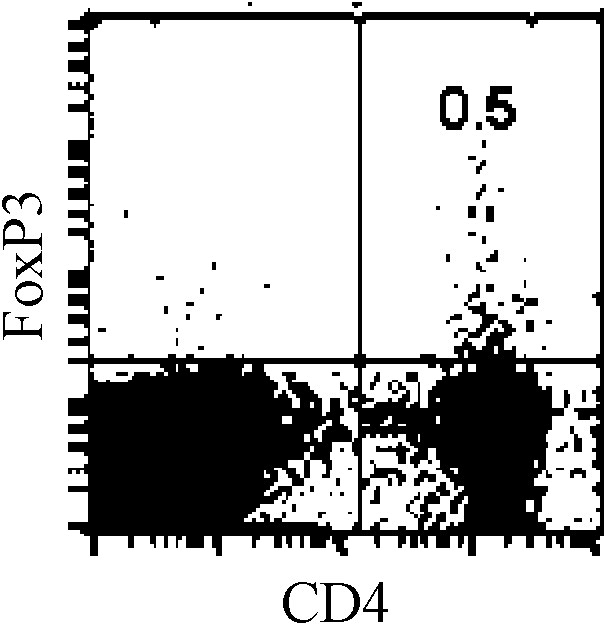
[0048] Example 3: Mouse FoxP3 + CD4 + Regulatory T cell induction assay
[0049] FoxP3 + CD4 + Regulatory T cells are a type of T cells with immunosuppressive effects. This type of cells participates in the regulation of immune response / immune tolerance in an "active" manner, not only participates in the regulation of autoimmune tolerance, but also plays an important role in transplantation immunity. In order to prove whether the formula I compound of the present invention can induce initial T cells to differentiate into FoxP3 + CD4 + Regulatory T cells, after the present invention isolates mouse splenocytes and stimulates them in vitro, FoxP3 is detected by flow cytometry (FACS) + CD4 + Differentiation of regulatory T cells. Specific steps are as follows:
[0050] According to the method of embodiment 1, be divided into control group, test group and blank group, cultivate with 24 well plate then, 1-2 * 10 6 / mL, 2mL / well. At 37°C, 5% CO 2 After incubating for 66 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com