Bis-o-vanillin ethylene diamine schiff base and transitional metal coordination compound and preparation method thereof
A technology of vanillin ethylenediamine schiff base and vanillin ethylenediamine, which is applied in the field of medicine, can solve problems such as no public reports and the like, and achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
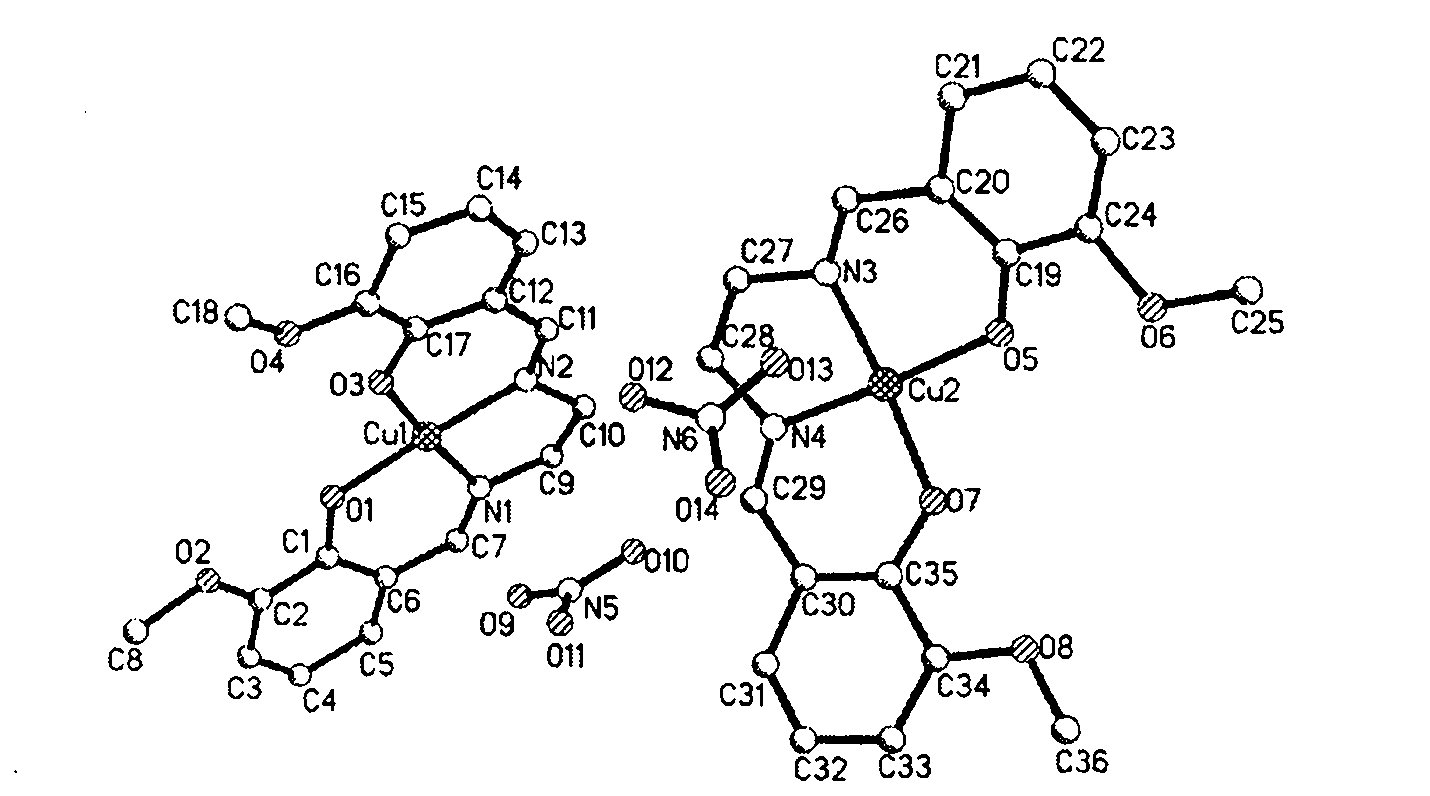
[0019] Embodiment 1, taking transition metal copper (Cu) as example, its molecular formula is {[Cu(C 18 h 18 N 2 o 4 )](NO 3 )(H 2 O)} complex preparation method is:
[0020] 0.0048g (0.02mmol) of Cu(NO 3 ) 2 Solid and 0.0075g (0.02mmol) of bis-o-vanillin ethylenediamine Schiff's base were placed in two clean vials respectively, 3mL and 5mL of methanol were added to dissolve it, and then the bis-o-vanillin The solution of ethylenediamine Schiffer's base was added dropwise to Cu(NO 3 ) 2 In the solution, after stirring evenly with a glass rod, filter the mixture into another clean vial, dilute it to 8mL with methanol, and let it stand at 18-25°C. The complex crystals will precipitate in 10-14 days, and the yield is 23 %. Infrared spectrum (KBr pellet, cm -1 ): 3436; 1636; 1385; 985; 740. UV-Vis absorption spectrum (in methanol), λmax / nm: 233.
Embodiment 2
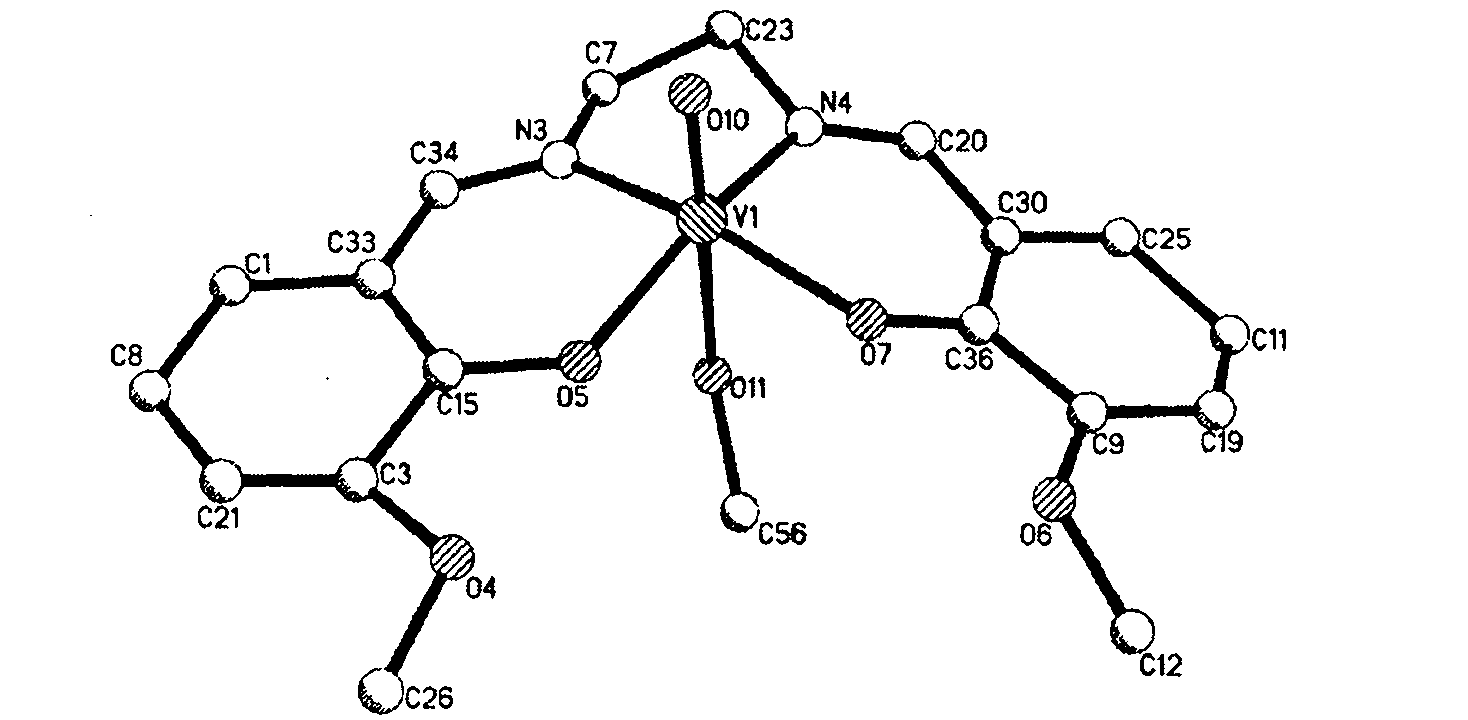
[0021] Embodiment 2, taking vanadium as example, molecular structural formula is {[V (C 18 h 18 N 2 o 4 )(CH 3 OH)(H 2 O)](H 2 O)} complex preparation method is:
[0022] Place 0.0075g (0.02mol) of bis-o-vanillin ethylenediamine Schiff's base in a vial, drop into 0.5mL N,N-dimethylformamide to dissolve it, then add 3mL of methanol; weigh 0.0032g (0.02mol) VOSO 4Put it in a vial, add 0.5mL N,N-dimethylformamide and heat to dissolve, then add 3mL methanol; then mix the two solutions, stir well, filter, and dilute the filtrate to 8mL with methanol, at 18-25℃ After standing still, crystals are formed in 10-14 days, and the yield is 30%. The melting point is 236-238°C. Infrared spectrum (KBr pellet, cm-1): 1591; 2929; 1440; 1302; 1246. UV-Vis absorption spectrum (in methanol), λmax / nm: 228.4; 294.3.
Embodiment 3
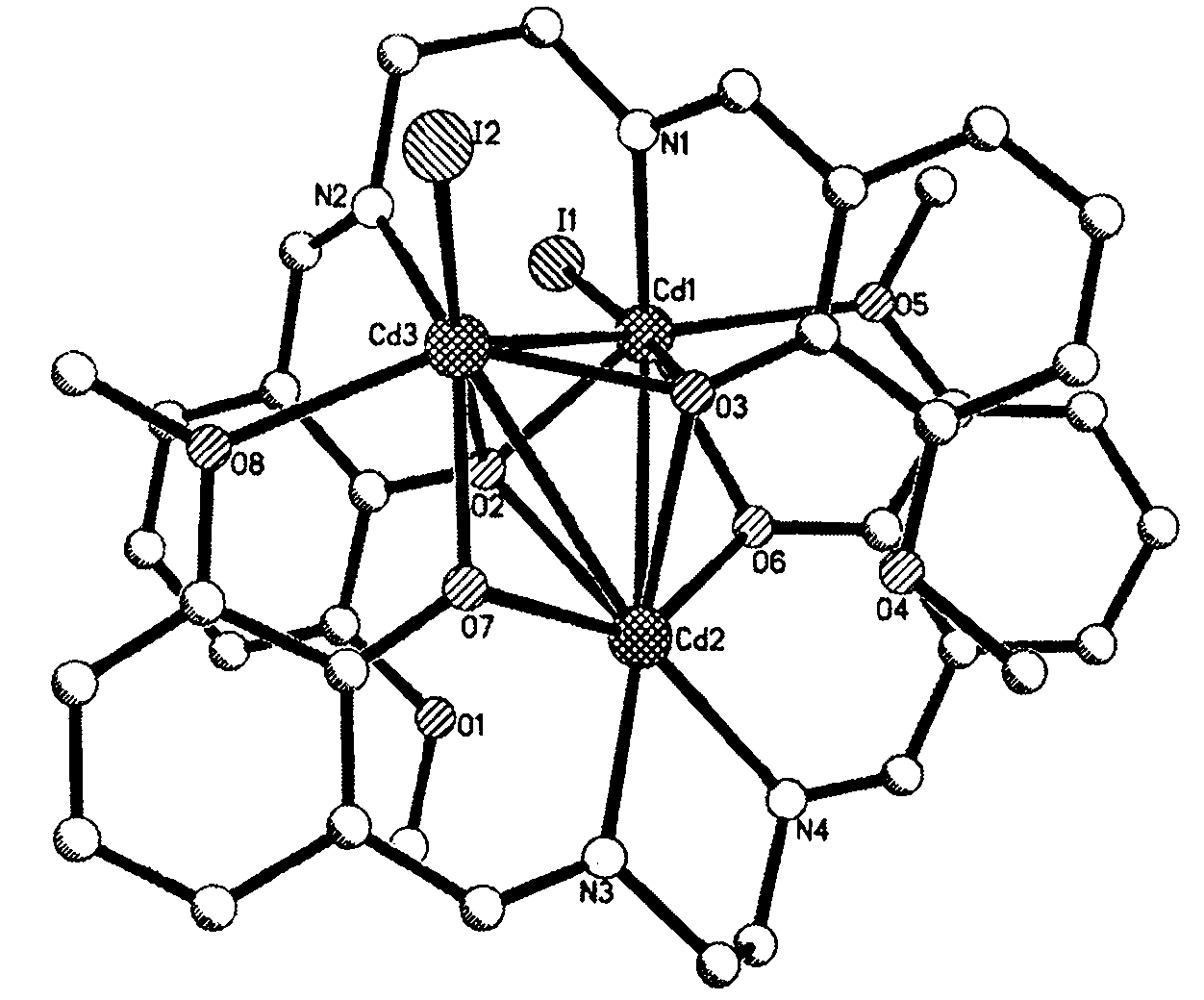
[0023] Embodiment 3, taking cadmium as example, molecular formula is [Cd 3 (C 18 h 18 N 2 o 4 ) 2 I 2 ] The compound preparation method is:
[0024] Accurately weigh 0.0075g (0.02mol) of bis-o-vanillin ethylenediamine Schiff's base in a vial, drop into 0.5mL N,N-dimethylformamide to dissolve it, and then add 3mL of methanol ; Weigh 0.0110g (0.03mol) CdI 2 Put it in a vial, add 0.5mL N,N-dimethylformamide and heat it to dissolve, then add 3mL methanol; then mix the two solutions, stir well, filter, dilute the filtrate to 8mL with methanol, in 18-25 After standing at ℃, pale yellow crystals are formed in 20-25 days, and the yield is 47%.
[0025] The present invention is used for the inhibitory action to α-glucosidase, and the method for its inhibition rate measurement is:
[0026] Take 4 plastic test tubes, add 1ml tris hydrochloric acid buffer solution of pH=7.20 and 1ml 10mmol L -1 The α-glucosidase (4-nitrophenyl-α-D-glucopyranosidase), the addition of other reagen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com