Pediatric anesthesia induction oral administration formulation and preparation method thereof
A technology of anesthesia induction and dosage forms, applied in the field of medicine, can solve the problems of increased incidence of complications, poor acceptance in children, increased regurgitation and aspiration, etc., and achieve a low probability of regurgitation and aspiration, good satisfaction, and firm attachment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1. Mix sucrose and maltose at a mass ratio of 1:1, heat and stir under normal pressure for about ten minutes to obtain a homogeneous and transparent sugar solution; the temperature of the sugar solution is controlled at about 180°C;
[0031] Step 2, select midazolam as the bulk drug, determine the amount of midazolam added according to the sedative dose, add the bulk drug midazolam to the sugar solution described in step 1, stir until the bulk drug is fully dissolved and mix evenly, to obtain a liquid sedative;
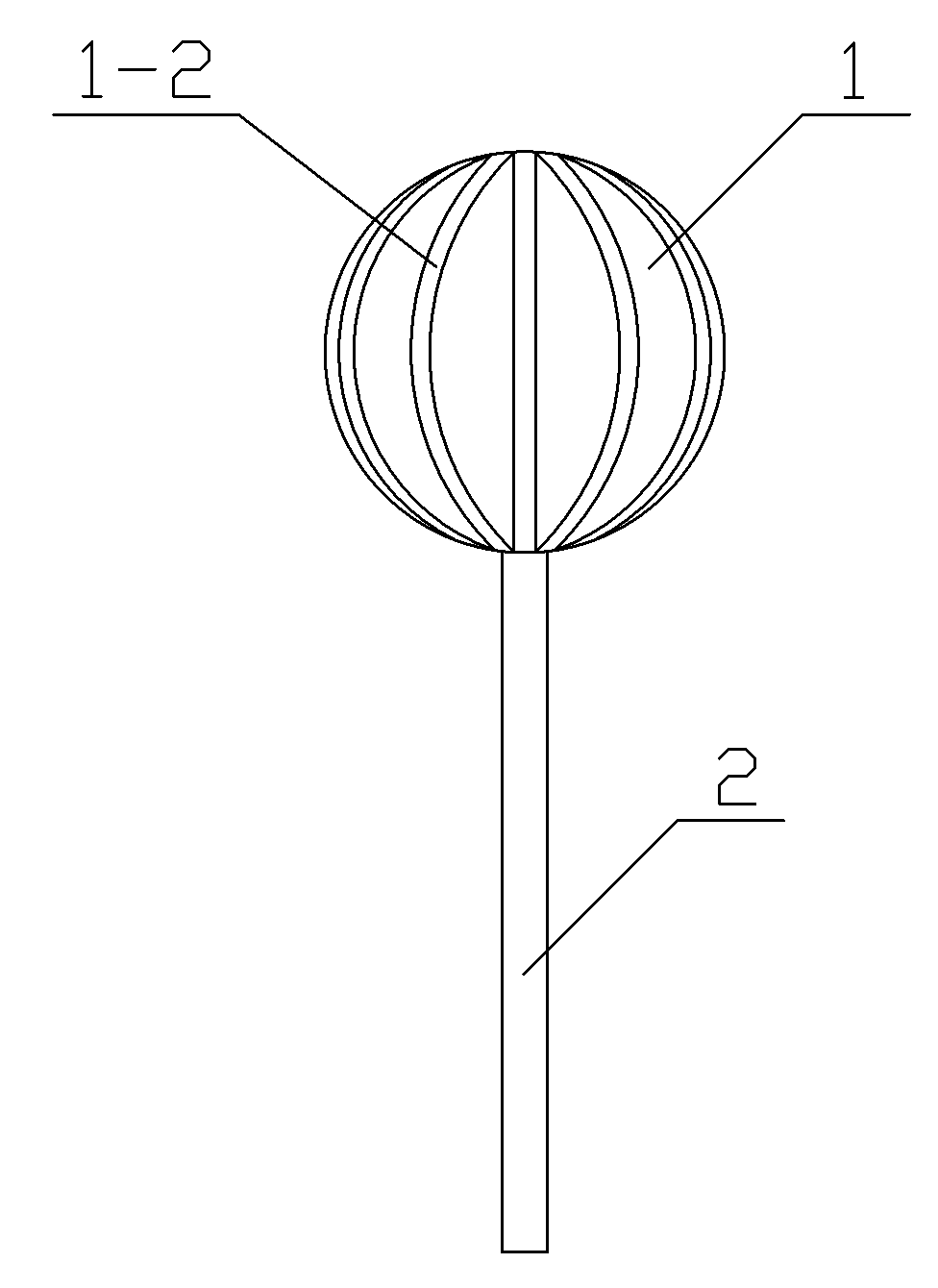
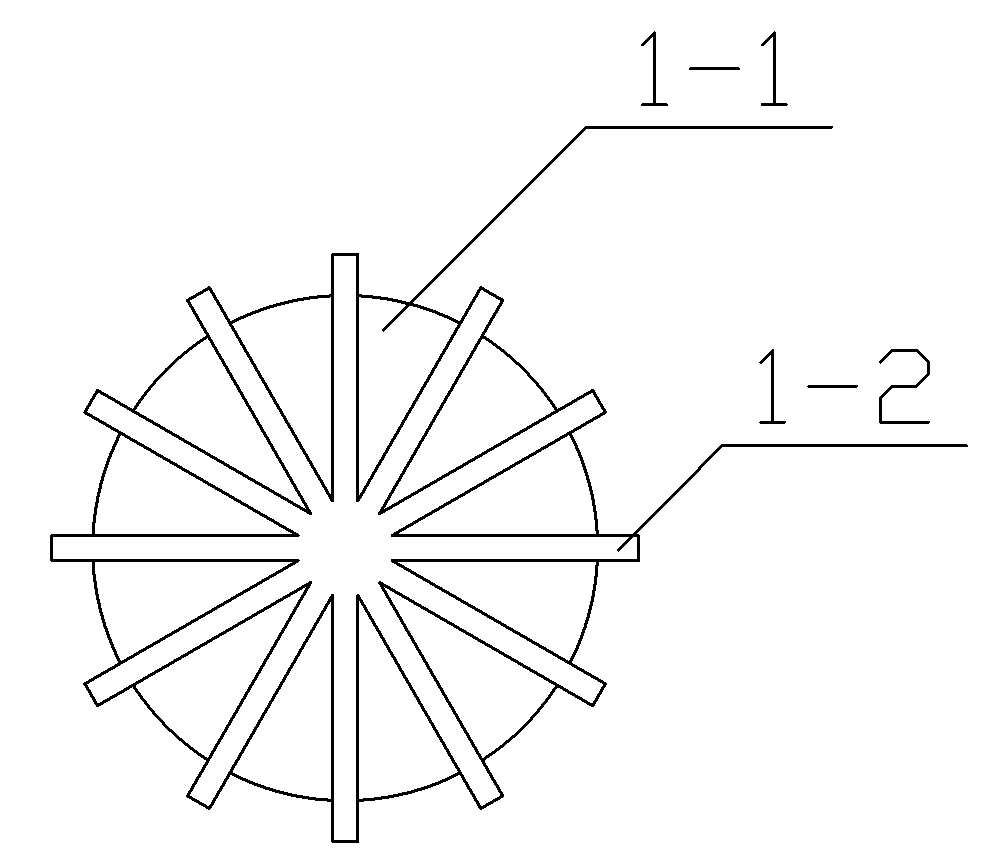
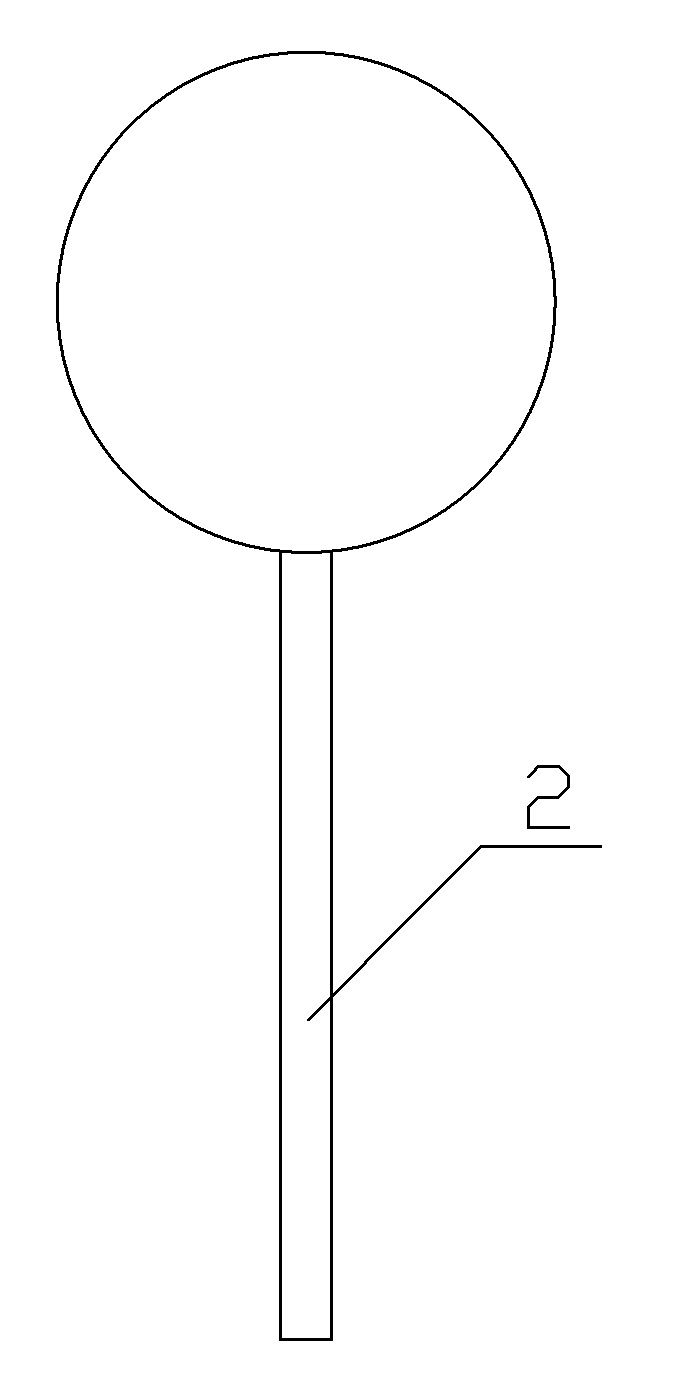
[0032] Step 3: Place the spherical plastic core 1 of the plastic core in the liquid sedative and rotate it so that the gap between the ridge-like protrusion stripes 1-2 of the spherical plastic core 1 is filled with liquid sedative, take it out after the sedative completely covers the ridge-like protrusion stripes, and continue Rotate until it is naturally cooled and formed to obtain a dosage form for pediatric anesthesia induction. The weight of the solid ...
Embodiment 2
[0042] Step 1. Mix sucrose and maltose at a mass ratio of 1:1, heat and stir under normal pressure for about ten minutes to obtain a homogeneous and transparent sugar solution; the temperature of the sugar solution is controlled at about 180°C;
[0043] Step 2, select dexmedetomidine as the bulk drug, determine the amount of dexmedetomidine added according to the sedative dose, add the bulk drug dexmedetomidine to the sugar solution described in step 1, and stir until the bulk drug is fully dissolved and mix evenly to obtain a liquid sedative;
[0044] Step 3: Place the spherical plastic core 1 of the plastic core in the liquid sedative and rotate it so that the gap between the ridge-like protrusion stripes 1-2 of the spherical plastic core 1 is filled with liquid sedative, take it out after the sedative completely covers the ridge-like protrusion stripes, and continue Rotating to natural cooling and molding to obtain a pediatric anesthesia induction buccal dosage form, the we...
Embodiment 3
[0054] Step 1. Mix sucrose and maltose at a mass ratio of 1:1, heat and stir under normal pressure for about ten minutes to obtain a homogeneous and transparent sugar solution; the temperature of the sugar solution is controlled at about 180°C;
[0055] Step 2. Select ketamine as the raw material drug, determine the amount of ketamine added according to the sedative dose, add the raw drug ketamine into the sugar solution described in step 1, stir until the raw material drug is fully dissolved and mix evenly to obtain a liquid sedative;
[0056] Step 3: Place the spherical plastic core 1 of the plastic core in the liquid sedative and rotate it so that the gap between the ridge-like protrusion stripes 1-2 of the spherical plastic core 1 is filled with liquid sedative, take it out after the sedative completely covers the ridge-like protrusion stripes, and continue Rotate until it is naturally cooled and molded to obtain a pediatric anesthesia induction buccal dosage form. The weight...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com