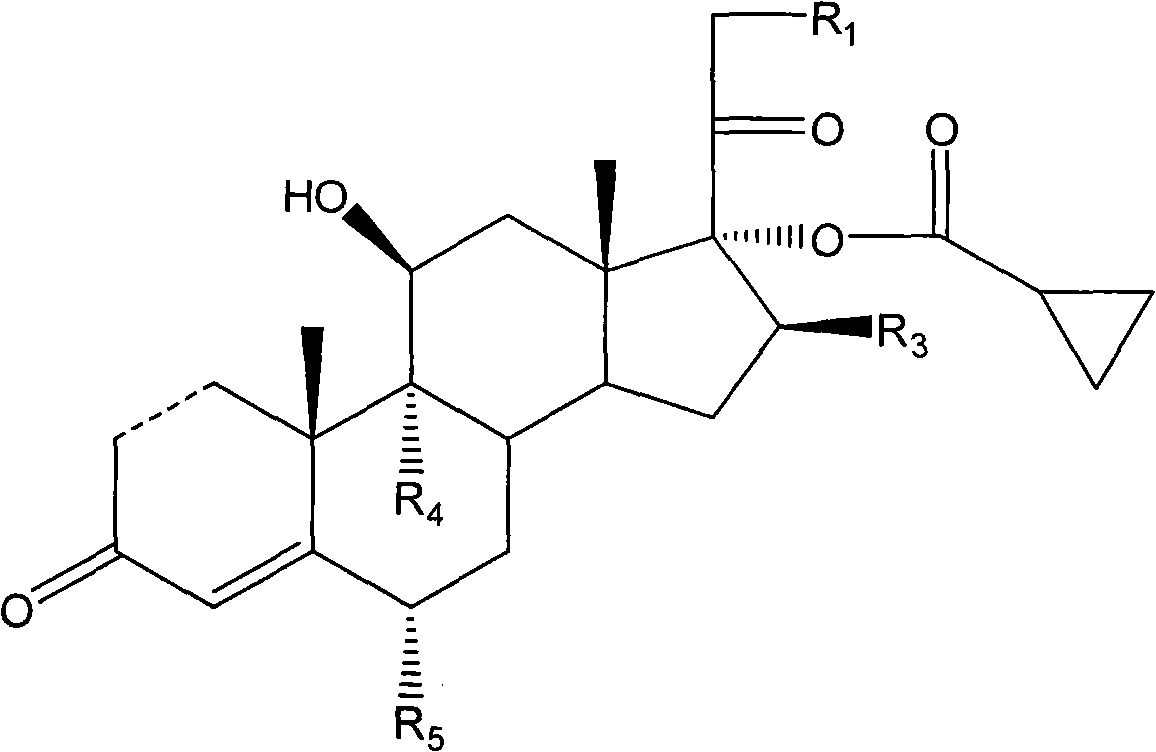
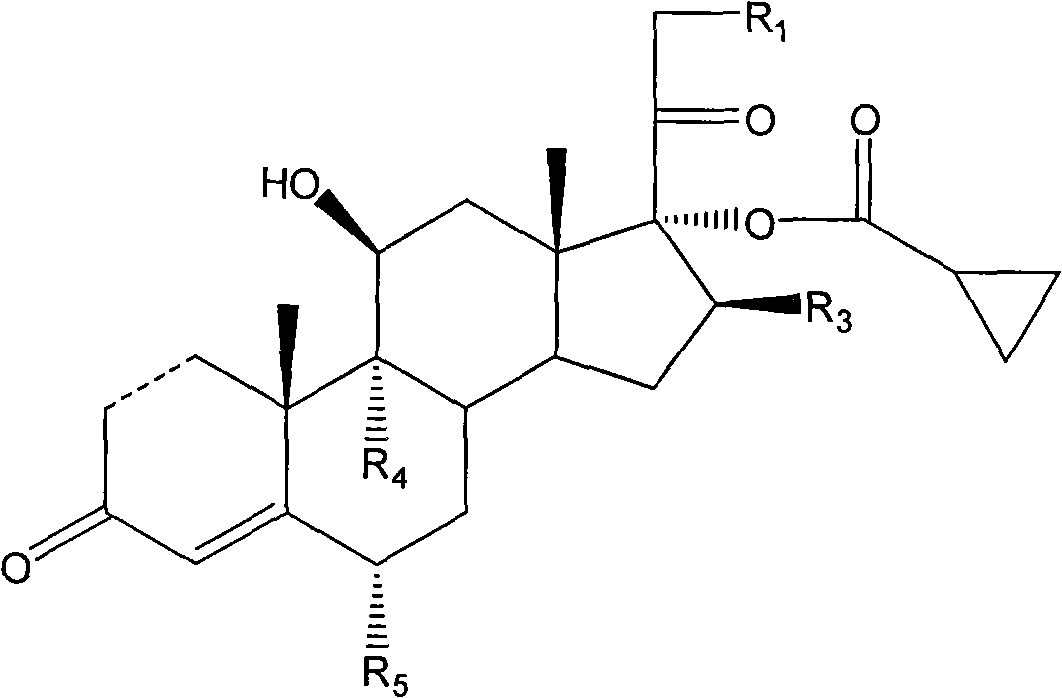
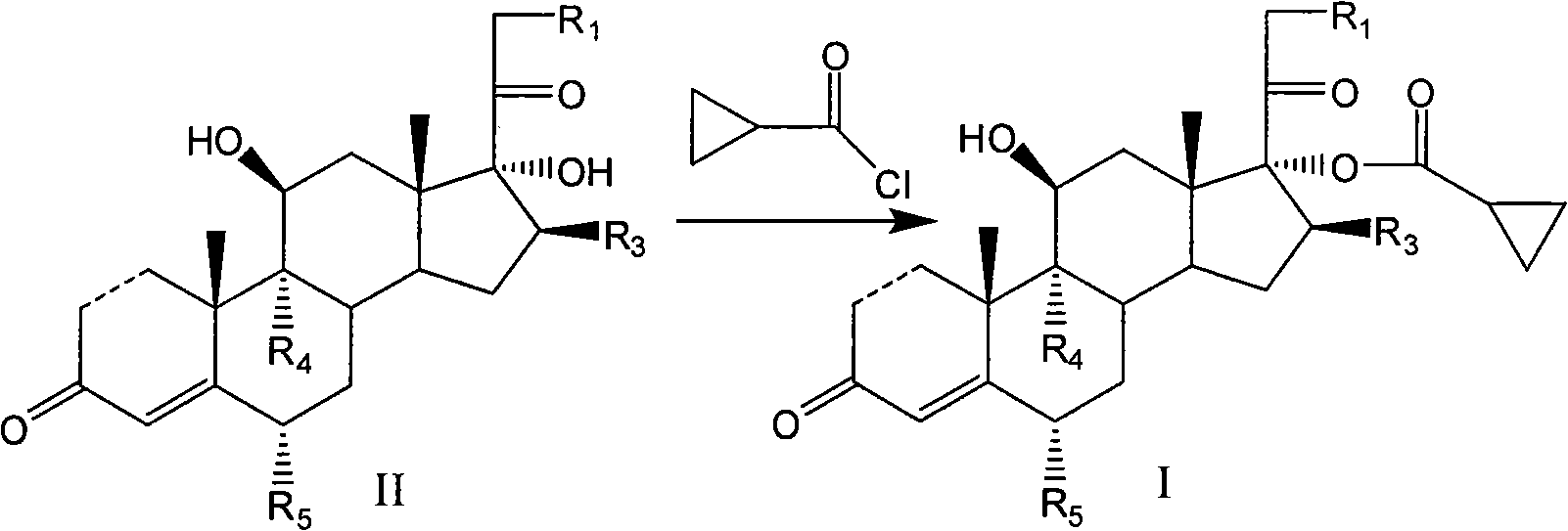
Cyclopropyl pregnene compound and application thereof
A compound, the technology of cyclopropyl formate, which is applied in the field of cyclopropyl-pregnant steroid glucocorticoids, can solve the problem of reducing adverse reactions and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0121] Example 1: 9α-fluoro-11β,17α,21-trihydroxy-16β-methyl-pregna-1,4-diene-3,20-diketone-17-cyclopropylcarboxylate-21- Preparation of acetate
[0122]
[0123] Dissolve 10mmol of 9α-fluoro-11β,17α,21-trihydroxy-pregna-1,4-diene-3,20-diketone-21-acetate in 50ml of pyridine, and cool the resulting solution to 0 ~5°C, add 0.2g of 4,4-dimethylaminopyridine, then slowly add 12mmol of cyclopropylformyl chloride at a temperature of 5-10°C, and then stir the obtained product at a temperature of 5-10°C The mixture was reacted for 4 hours with 2N hydrochloric acid added dropwise to adjust the pH value to 2-3, diluted in ice water, and the aqueous phase was extracted three times with dichloromethane, the dichloromethane was combined, and the pH was adjusted to 5-3 with sodium bicarbonate solution. 6 and washed with water, the organic phase was concentrated, and recrystallized from methanol to obtain 4.2 mmol of the target compound.
[0124] Elemental analysis measured value: C28H...
Embodiment 2
[0127] Example 2: Preparation of 9α-fluoro-11β, 17α, 21-trihydroxy-16β-methyl-pregna-1,4-diene-3,20-diketone-17-cyclopropyl carboxylate
[0128]
[0129]The pH in Example 1 is 5-6 and the methylene chloride phase washed with water is added dropwise to the organic phase with 2% NaOH / methanol solution at 0-5 degrees under nitrogen, and after reacting for 3 hours, use acetic acid to adjust the pH 6-7, 3.2 mmol of the target compound was obtained by column chromatography.
[0130] Elemental analysis measured value: C26H33FO6 C, 67.78; H, 7.21; F, 4.14; O, 20.87
Embodiment 3
[0131] Example 3: Preparation of Pregna-1,4-diene-3,20-diketone-11,17,21-trihydroxy-17-cyclopropylcarboxylate-21-acetate
[0132]
[0133] Using pregna-1,4-diene-3,20-diketone-11,17,21-trihydroxy-21-acetate as raw material, 5.2 mmol of the target compound was synthesized according to the process of Example 1.
[0134] Elemental analysis measured value: C27H34O7 C, 68.92; H, 7.28; O, 23.80
[0135] 13 C-NMR: 13.2(CH), 8.4(CH2), 8.4(CH2), 101.9(C), 41.2(C), 50.3(CH), 22.9(CH2), 24.1(CH), 186.0(C), 68.9 (CH), 31.5(CH), 40.3(CH2), 168.2(C), 124.5(CH), 128.7(CH), 158.8(CH), 44.3(C), 59.2(CH), 32.3(CH2), 32.9 (CH2), 171.5(C), 170.4(C), 212.1(C), 68.0(CH2), 17.0(CH3), 19.1(CH3), 20.6(CH3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com