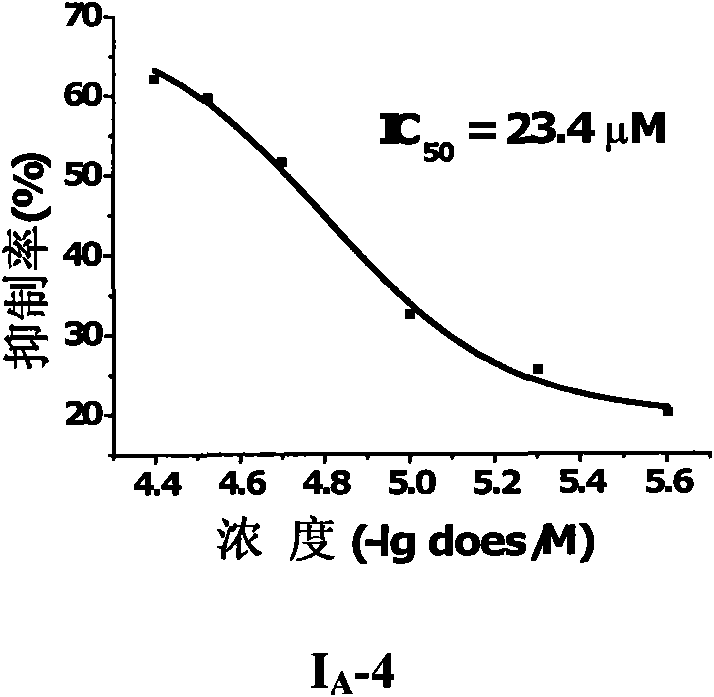
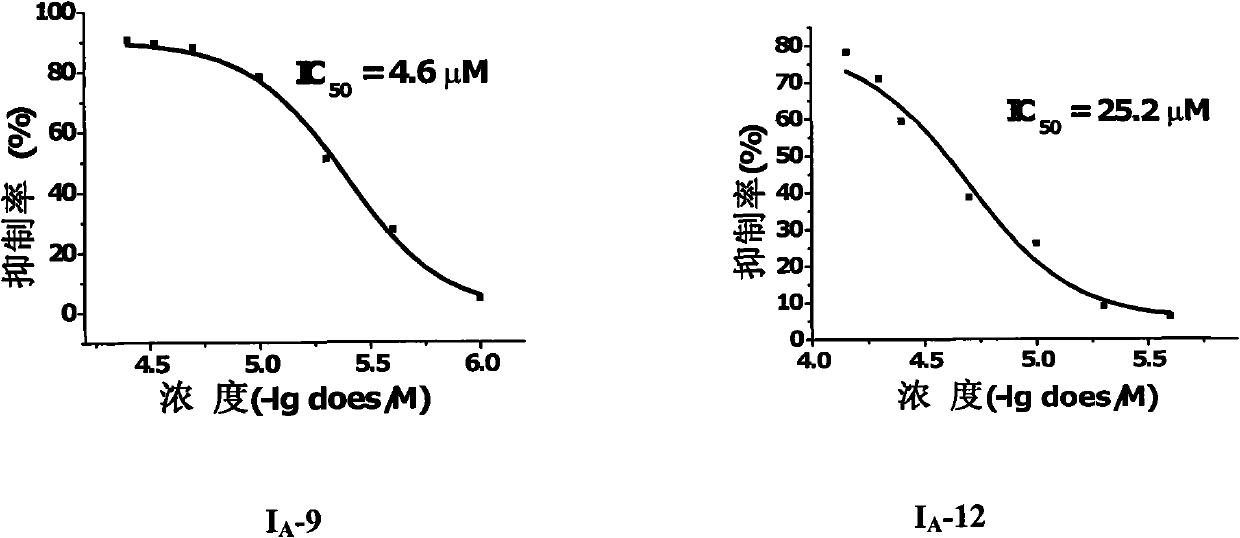
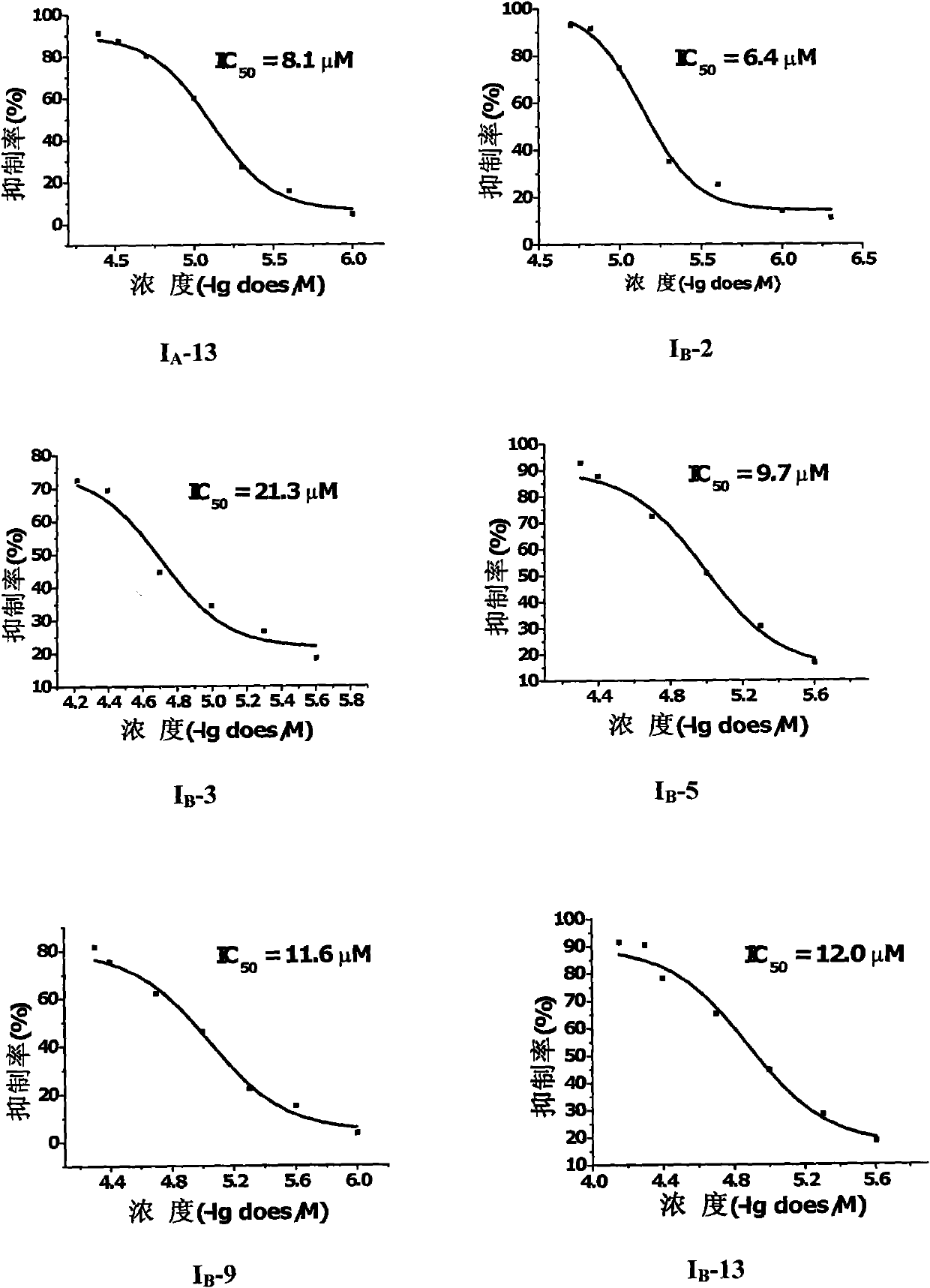
3-methoxy-4-(N-substituted amino sulfonyl)fenalamide compounds and application thereof
A technology based on phenylpropanamide and sulfamoyl, which is applied in the field of 3-methoxy-4-phenylpropanamide compounds and can solve problems affecting the function of oocysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Methyl 3-methoxyphenylpropionate (IV)
[0056] Dissolve 1.5 g of 3-methoxyphenylpropionic acid in an eggplant-shaped flask filled with 40 mL of anhydrous methanol, slowly add about 1 mL of concentrated sulfuric acid dropwise to the system with stirring at room temperature, and heat to 70°C for 5 hours under reflux. Cooled to room temperature, the methanol solvent in the system was distilled off under reduced pressure, followed by saturated NaHCO 3 The solution was washed, and the system was adjusted to pH=7. Extracted with ethyl acetate (20 mL×3), combined the obtained organic layers, and washed with distilled water (10 mL×3). The organic layer was dried with anhydrous magnesium sulfate, suction filtered, and the solvent was distilled off under reduced pressure to obtain 1.4 g of yellow oil (IV), with a yield of 87%.
Embodiment 2
[0058] Methyl 3-methoxy-4-chlorosulfonylphenylpropionate (V)
[0059] 1 mL of chlorosulfonic acid was slowly added dropwise to an eggplant flask containing 1.4 g of methyl 3-methoxyphenylpropionate. Control the rate of addition, keep the temperature of the reaction solution not higher than 40°C, stir for 5 hours, pour the reaction system into a beaker filled with ice cubes, extract with ethyl acetate (20mL × 3), mix the organic layer obtained, and then Wash with distilled water (10 mL×3). The organic layer was dried with anhydrous magnesium sulfate, suction filtered, and the solvent was distilled off under reduced pressure to obtain 1.4 g of light yellow oil (V), with a yield of 66%. 1 H-NMR (400Hz, CDCl 3 ), δ: 2.77(2H, t), 3.43(2H, t), 3.71(3H, s), 3.90(3H, s), 6.89(1H, d), 6.95(1H, d), 8.04(1H, d).
Embodiment 3
[0061] Methyl 3-methoxy-4-(N-(2-methyl-4-nitro)sulfamoyl)phenylpropionate (VI)
[0062] Dissolve 0.78 g of 2-methyl-4-nitroaniline in 20 mL of distilled anhydrous tetrahydrofuran, and add 2 mL of anhydrous pyridine, then add 1.4 g of 3-methoxy-4-chlorosulfonylphenylpropionic acid A solution of methyl ester (V) in anhydrous tetrahydrofuran (10 mL) was added dropwise to the above system. The whole reaction system N 2 Reflux overnight at 70°C under protection. The reaction was monitored by thin-layer chromatography, and after the reaction of the raw material (V) was complete, it was cooled to room temperature. Tetrahydrofuran in the system was distilled off under reduced pressure, dissolved in dichloromethane, and then washed with distilled water (20 mL×3) to remove a small amount of residual pyridine. The dichloromethane layer was dried with anhydrous magnesium sulfate, suction filtered, and the solvent was distilled off under reduced pressure, and the obtained oily mixture w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com