Method for improving in-vitro expression of cytochrome P450 enzyme family
A technology of in vitro expression and cytochrome, applied in botany equipment and methods, biochemical equipment and methods, plant gene improvement, etc., can solve problems such as difficult to meet research needs and low yield, and achieve good social benefits and considerable economic benefits benefit effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Construction and identification of recombinant transfer vector pFastBac1-CYP2A13 plasmid
[0034] pFastBac1-CYP2A13 is preserved in our laboratory. The Escherichia coli containing the recombinant plasmid was obtained by screening on the ampicillin resistance plate, and the DNA of the bacterial solution was extracted to obtain the plasmid DNA of the recombinant transfer vector. Validation of pFastBac1-CYP2A13 using double restriction digestion.
[0035] 2. Construction and identification of recombinant eukaryotic expression virus Ac-Bacmid-CYP2A13 DNA
[0036] The constructed pFastBac1-CYP 2A13 recombinant plasmid was transformed into DH10Bac Escherichia coli competent cells, and with the assistance of the helper plasmid Helper of DH10Bac Escherichia coli, transposoned by Tn7 transposon, each CYP2A13 gene fragment was inserted into the shuttle vector Bacmid, and Kanamycin, gentamicin and tetracycline three-antibody screening and blue-white screening to obtain recomb...
Embodiment 2
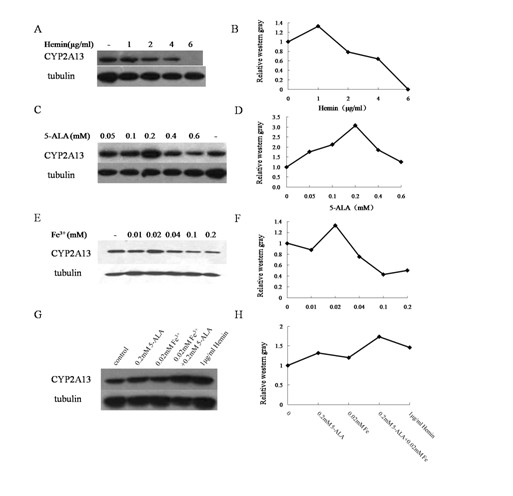
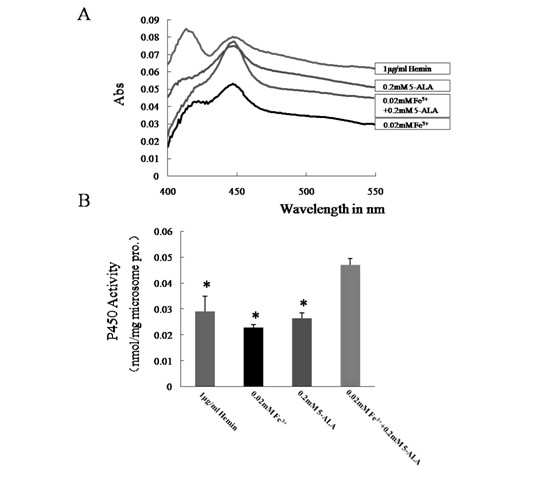
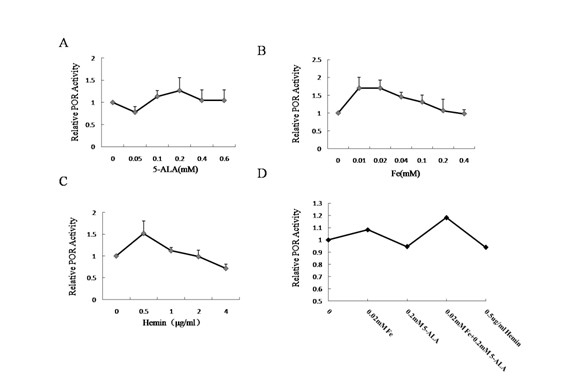
[0043] Compared with Example 1, except that the cofactor in step 4 is 0.05-0.6mM 5-ALA, the others are the same.
Embodiment 3
[0045] Compared with Example 1 except that the cofactor in step 4 is 0.01-0.4mM Fe 3+ Except for the difference, everything else is the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com