Increased production of aspartic proteases in filamentous fungal cells
A filamentous fungus and cell technology, applied in the field of increasing the production of aspartic protease in filamentous fungal cells, can solve the problems of interfering with the effective production of heterologous proteins and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
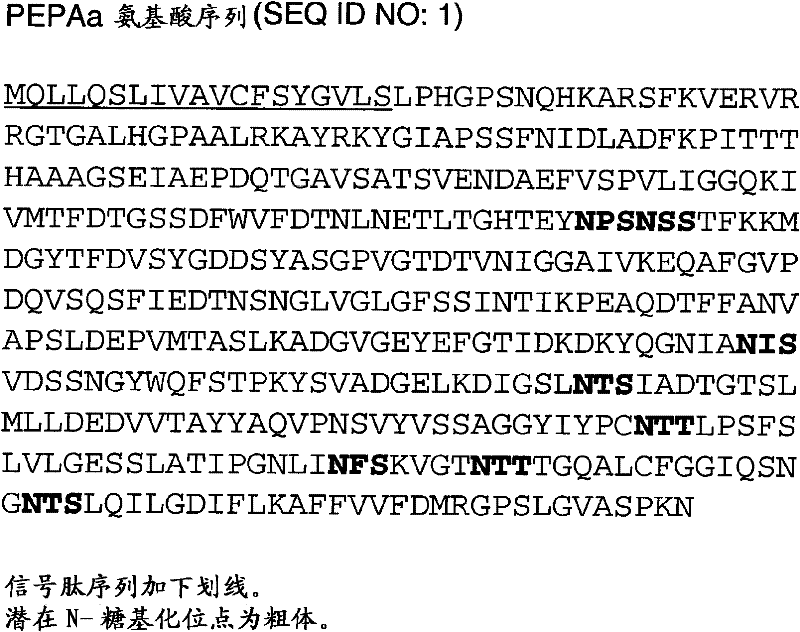
[0253] Construction of recombinant pepAa gene and production of PEPAa by the transformed GAP3 strain of Aspergillus niger protein
[0254] The pepAa gene encodes a 424 amino acid PEPAa protein (SEQ ID NO: 1), which has a 19 amino acid signal sequence. This example illustrates: (1) Construction of the plasmid vector pGAMD-pepAa, which has a recombinant gene of pepAa inserted between the Aspergillus niger glucoamylase promoter and the Aspergillus tubing glucoamylase terminator; (2) The vector was used to transform Aspergillus niger to obtain the integrated recombinant gene; (3) selecting Aspergillus niger strains overexpressing pepAa; and (4) measuring the PEPAa protein production of the overexpressing strains.
[0255] In order to construct the recombinant expression plasmid of the Aspergillus niger pepAa gene, two primers CACTCGAGGCCACCATGCAGCTCCTCCAG (SEQ ID NO: 5) and AGGAAACTAGTTCTTGGGAGAGGCAAC (SEQ ID NO: 6) were used in the Pfu PCR reaction containing the genomic DNA templat...
Embodiment 2
[0275] Construct the recombinant truncated pepAa gene and transform it into the GAP3 strain of Aspergillus niger
[0276] Preparation of recombinant truncated form of pepAa gene ("pepAa * ”) and transformed into the GAP3 strain of Aspergillus niger to prove the importance of this sequence for the secretion of the full-length wild-type PEPAa protein (SEQ ID NO:1). The full-length pepAa gene has the coding position 382 in SEQ ID NO:1 The 3'nucleotide sequence of the following C-terminal amino acid starting at:
[0277] LCFGGIQSNGNTSLQILGDIFLKAFFVVFDMRGPSLGVASPKN.
[0278] Such as Figure 24 Shown by pepAa * Encoded PEPAa * The amino acid sequence of the truncated form of the protein (SEQ ID NO: 23) does not have the C-terminal 43 amino acids of SEQ ID NO: 1, but has 13 different amino acids inserted. Therefore, PEPAa and PEPAa * The amino acid sequence at positions 1-381 are the same, but starting from position 382, PEPAa * The sequence (SEQ ID NO: 23) has a C-terminal amino acid: ...
Embodiment 3
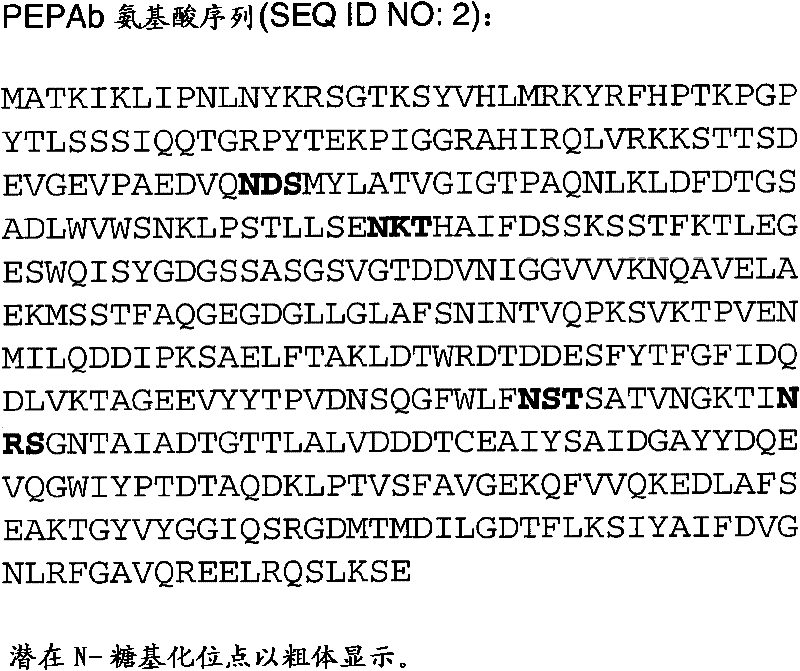
[0284] Construction of recombinant pepAb gene and production of PEPAb egg by transformed Aspergillus niger GAP3 strain White matter
[0285] The recombinant expression plasmid of the Aspergillus niger pepAb gene was constructed as described for the pepAa gene in Example 1, except that the two primers used in the Pfu PCR reaction containing the genomic DNA template obtained from the UVK143 strain were AACTCGAGTCCATCATGGCTACCAAAATC (SEQ ID NO: 10 ) And CCTCTAGACTACTCCGACTTCAGGCTC (SEQ ID NO: 11). The 1418bp nucleotide sequence of the resulting PCR amplicon (SEQ ID NO: 12) is shown in Picture 10 in.
[0286] The PCR amplicon (SEQ ID NO: 12) was cloned into the pGAMD vector (as described for pepAa amplicon in Example 1). The resulting plasmid pGAMD-pepAb (shown in Picture 11 Medium) contains a recombinant gene including pepAb inserted between the Aspergillus niger glucoamylase promoter and the Aspergillus tubing glucoamylase terminator.
[0287] As in Example 1, the pGAMD-pepAb pla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com