Carbohydrate antigen (CA) 125 chemiluminescent quantitative test kit
A technology for quantitative detection of sugar chain antigens, applied in chemiluminescence/bioluminescence, biological testing, and analysis through chemical reactions of materials, can solve the problems of difficult detection of endometrial cell carcinoma and clear cell carcinoma, and achieve The effects of retaining high specificity, improving sensitivity, and accurate detection results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Preparation of CA19-9 Quantitative Detection Kit
[0024] The main steps are: (1) Anti-CA125 antibody-coated plate preparation (CA125 detection reaction plate preparation); (2) Enzyme-labeled antibody preparation; (3) CA125 calibration product preparation; (4) CA125 quality control product (5) Preparation of chemiluminescent substrate solution; (6) Preparation of washing solution; (7) Composition of semi-finished products and finished products
[0025] (1) Anti-CA125 antibody-coated plate preparation
[0026] a. Coating: Take 1mol / L Na 2 HPO 4 77.4ml and 1mol / L NaH 2 PO 4 22.6ml, mix evenly, add deionized water to 1000ml to form a 10-fold coating solution, dilute ten times before use, add an appropriate amount of anti-CA125 monoclonal antibody (purchased from Sweden Cornell) and mix well, then add to the microwell In plate wells, 100 μl / well, 4°C for 16 hours;
[0027] b. Sealing: Discard the coating solution, pat dry on absorbent paper, add 3% BSA and 0.05% pre...
Embodiment 2
[0043] How to use the CA125 quantitative detection kit
[0044] 1. Reagent and Sample Preparation
[0045] (1) Reagent preparation
[0046] a. Put the kit at room temperature (18-26°C) to equilibrate for 20 minutes.
[0047] b. Take out the concentrated washing solution from the kit, dilute it 1:20 with fresh purified water and add it to the washing solution bottle of the plate washer.
[0048] (2) Sample preparation
[0049] The qualified serum or plasma to be tested should be equilibrated at room temperature (18-26°C) for 20 minutes before use.
[0050] 2. Operation steps
[0051] a. Take out the required CA125 antibody-coated microwell plate and place it on the microwell rack
[0052] b. Add calibrators 1-6, quality control products 1-2 and samples to be tested, 50 μl per well, mix thoroughly, and incubate in a 37°C incubator for 90 minutes.
[0053] c. Manual washing: Discard the liquid in the wells, fill each well with the washing liquid, let it stand for 5 seconds,...
Embodiment 3
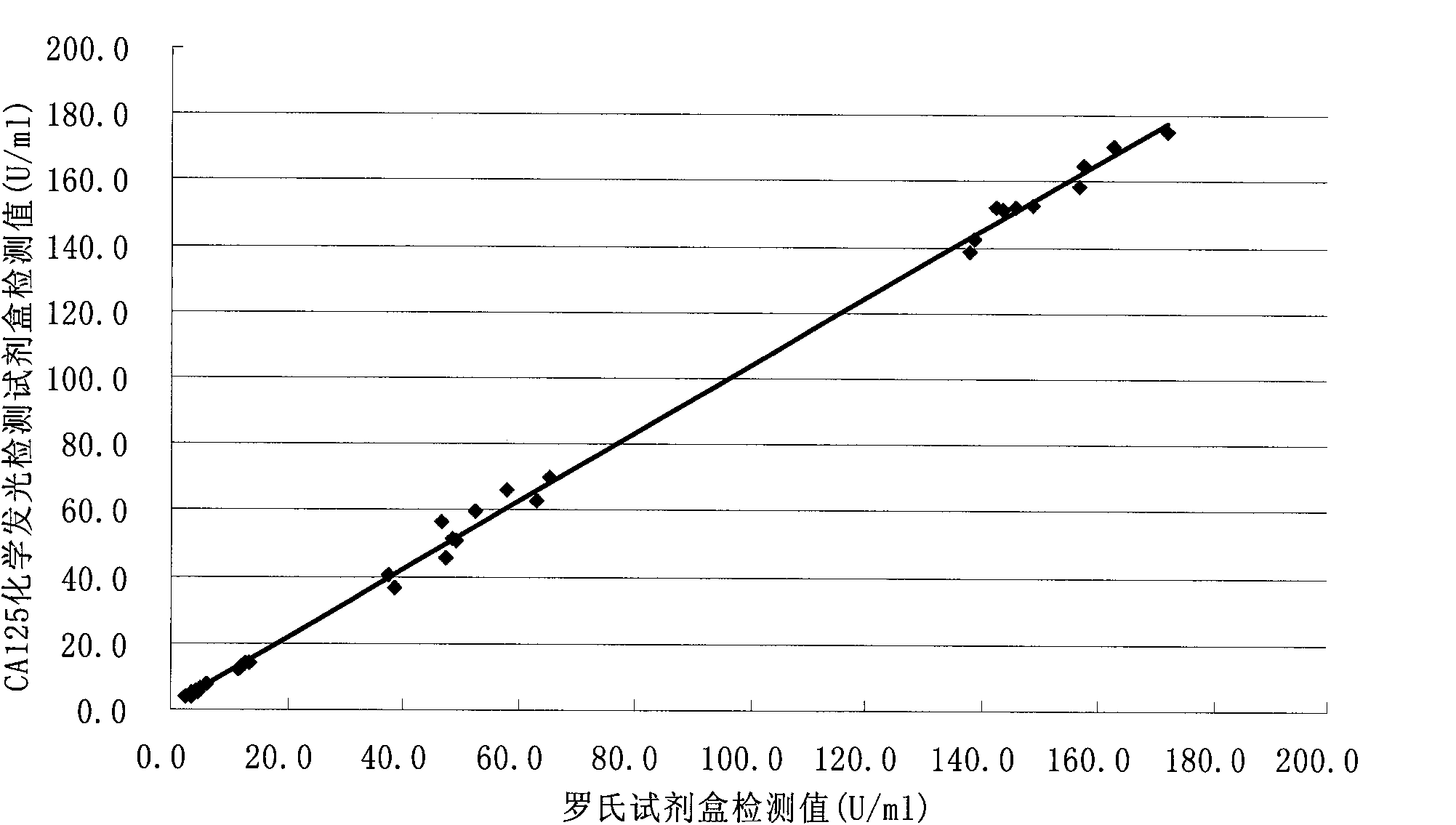
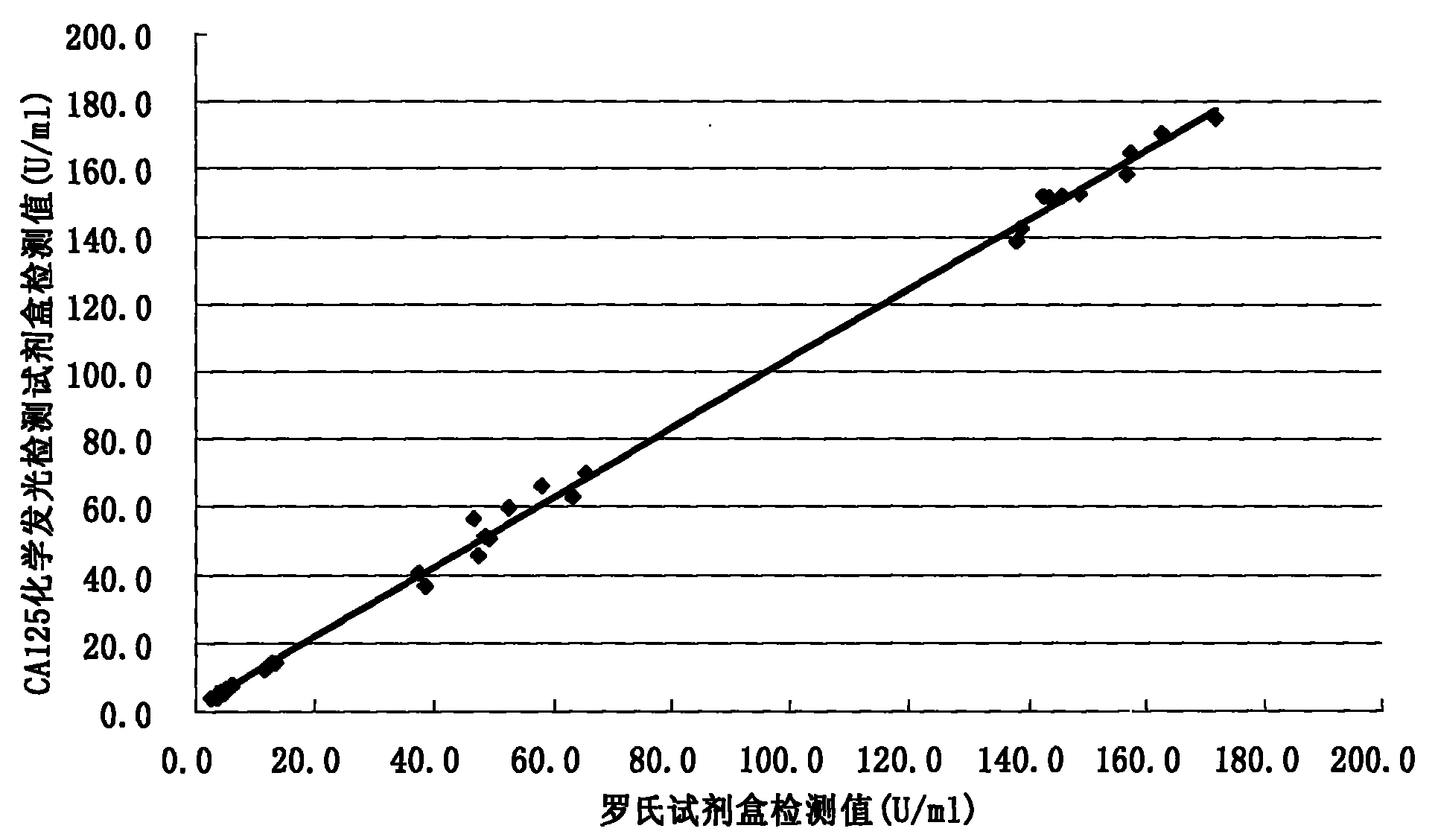
[0062] Methodological Identification of CA125 Quantitative Detection Kit
[0063] According to the conventional manufacturing and identification procedures in the art, the test kit prepared in Example 1 is tested and the results are as follows:
[0064] 1. Accuracy
[0065] The known content sample is detected with the kit prepared in Example 1 of the present invention, and the recovery rate is calculated (recovery rate=recovery amount / addition amount×100%), the sample size is not less than 10, and the results are as follows:
[0066] Amount added
[0067] 2. Specificity
[0068] The known content analogue is detected with the kit prepared in the embodiment of the present invention, and the cross-reaction rate is calculated (cross-reaction rate=detection value / addition amount×100%), and the results are as follows:
[0069]
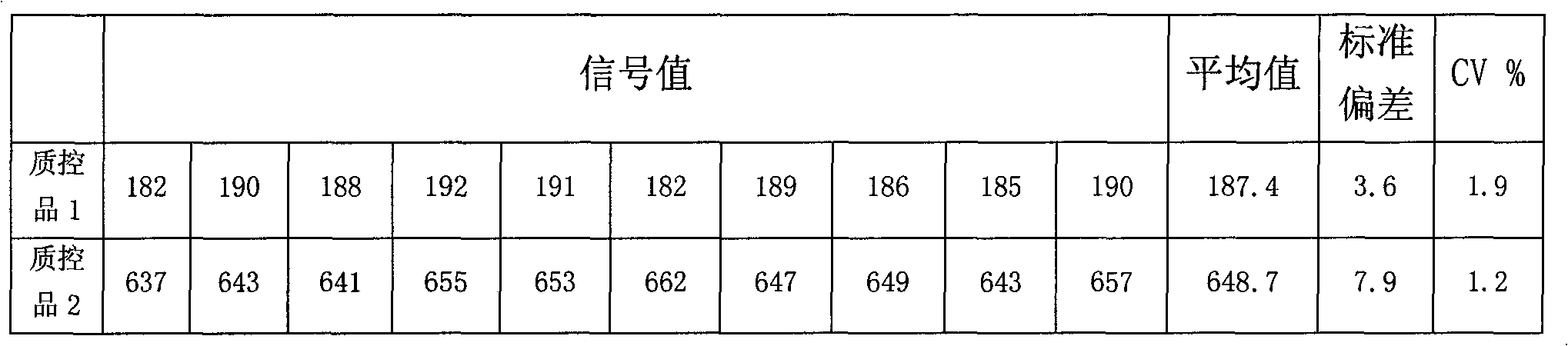
[0070] 3. Precision
[0071] Make 10 parallel wells of quality control product 1 and quality control product 2 respectively, and ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com