Protein chip for detecting blood and cerebro spinal fluid pathogen antibody, and its preparing method and use
A pathogen antibody and protein chip technology, which is applied in biochemical equipment and methods, measuring devices, and microbial determination/inspection. Large and other problems, to achieve the effect of retaining a high degree of specificity, improving detection speed and efficiency, and using less sample
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
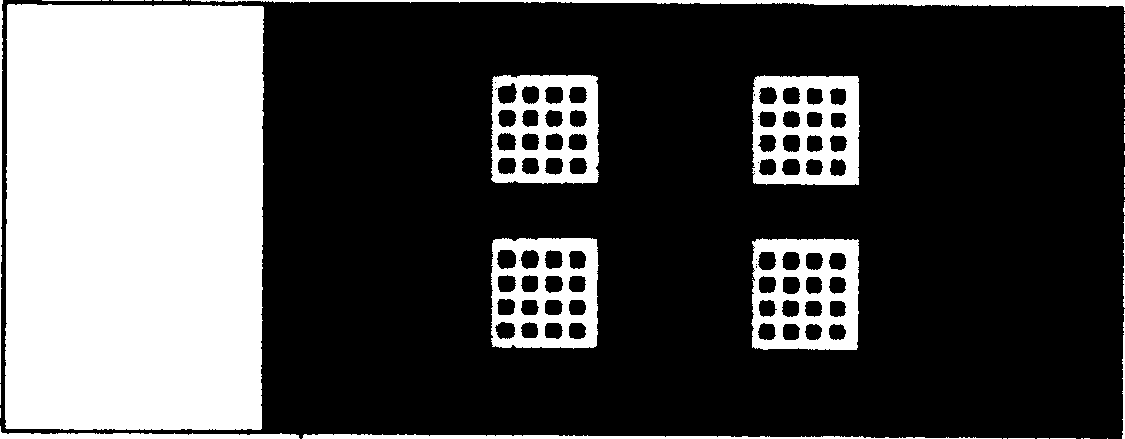
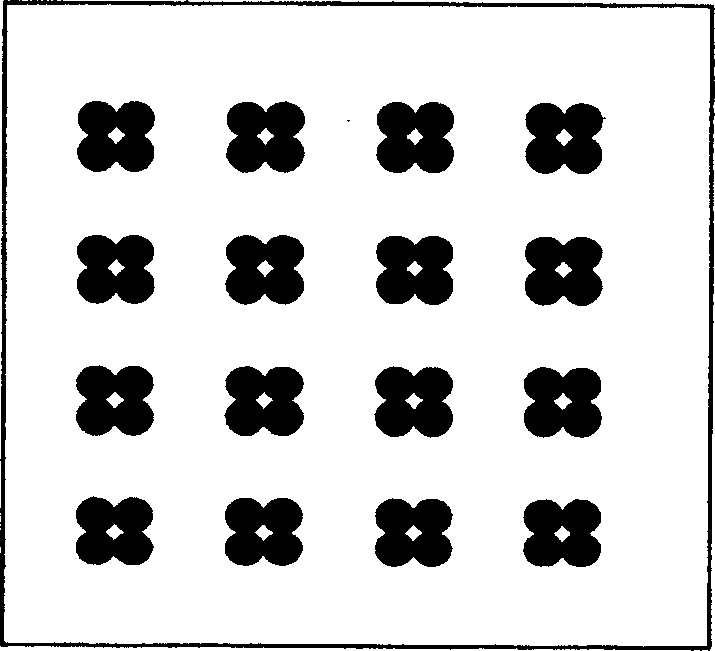
[0046] Example 1: Modification of slides and immobilization of antigens, such as figure 1 To detect the appearance of the antibody protein chip of serum cerebrospinal fluid pathogens, such as figure 2 Spot array diagram.
[0047] Place the slide on the slide rack, put it into a glass jar containing 350ml cleaning solution (NaOH 100g, ethanol 600ml, water 400ml), place it on a shaker at 60 rpm, shake it for 2 hours; discard the cleaning solution, Wash 4 times with water for 3 minutes each time; soak the slides in a glass jar filled with 350ml polylysine PBS solution (polylysine 35ml, PBS 35ml, water 280ml), and set a shaker at 60 rpm , Shake for 1 hour; immerse the slides in water and wash 5 times up and down; put in a centrifuge, 800 rpm / 5 minutes after separating the core, put it in a clean plastic box, place it vertically for 2 weeks or place the sample after baking slices in the oven use.
[0048] Do 2 serum samples testing, choose 2×2 microarray chip, (such as figure 1 ) ...
Embodiment 2
[0050] Example 2: Antigen-antibody reaction and detection
[0051] Add blocking solution (1% BSA, 0.2g / L KCl, 1.44g / L Na2HPO4, 0.24g / L KH2PO4, 8g / L NaCl, 0.1% Tween-20,) to block on the chip with good antigen, 37℃1 Hours, block non-specific sites on the substrate surface; wash with PBST (0.2g / L KCl, 1.44g / L Na2HPO4, 0.24g / L KH2PO4, 8g / L NaCl, 0.1% Tween-20) 3 times, 10 times each time After seeding seconds, rinse with PBS (0.2g / L KCl, 1.44g / L Na2HPO4, 0.24g / L KH2PO4, 8g / L NaCl), centrifuge at 800 rpm / centrifuge for 3 minutes to remove excess blocking solution; Dilute 10 times with PBS and add 3μL to the array. The first sample is added to the upper and lower arrays in the first column; the second sample is added to the upper and lower arrays in the second column and placed in the hybridization box at 37℃ 30 minutes to make the antigen and antibody fully react; wash 3 times with PBST, after 10 seconds each time, rinse with PBS, centrifuge at 800 rpm / centrifuge for 3 minutes to remo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com