Chemiluminescence quantitative detection kit for carbohydrate antigen 19-9
A technology for quantitative detection of sugar chain antigens, applied in chemiluminescence/bioluminescence, analysis through chemical reactions of materials, measurement devices, etc., can solve the problems of weak and elevated organ specificity, and achieve specificity retention , Accurate detection results, and the effect of improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of CA19-9 Quantitative Detection Kit
[0020] The steps are mainly: (1) preparation of anti-CA19-9 antibody-coated plate (preparation of CA19-9 detection reaction plate); (2) preparation of enzyme-labeled antibody; (3) preparation of CA19-9 calibrator; ( 4) Preparation of CA19-9 quality control products; (5) Preparation of chemiluminescent substrate solution; (6) Preparation of washing solution; (7) Composition of semi-finished products and finished products
[0021] (1) Preparation of anti-CA19-9 antibody-coated plates
[0022] a. Coating: Take 1mol / L Na 2 HPO 4 77.4ml and 1mol / L NaH 2 PO 4 22.6ml, mix evenly, add deionized water to 1000ml to form a 10-fold coating solution, dilute ten-fold before use, add an appropriate amount of anti-CA19-9 monoclonal antibody (purchased from Kangnaig, Sweden) and mix evenly, and then add to In microplate wells, 100 μl / well, 4°C for 16 hours;
[0023] b. Sealing: discard the coating solution, pat dry on absorbent ...
Embodiment 2
[0039] How to use the CA19-9 quantitative detection kit
[0040]1. Reagent and Sample Preparation
[0041] (1) Reagent preparation
[0042] a. Put the kit at room temperature (18-26°C) to equilibrate for 20 minutes.
[0043] b. Take out the concentrated washing solution from the kit, dilute it 1:20 with fresh purified water and add it to the washing solution bottle of the plate washer.
[0044] (2) Sample preparation
[0045] The qualified serum or plasma to be tested should be equilibrated at room temperature (18-26°C) for 20 minutes before use.
[0046] 2. Operation steps
[0047] a. Take out the required CA19-9 antibody-coated microwell plate and place it on the microwell rack
[0048] b. Add calibrators 1-6, quality control products 1-2 and samples to be tested, 50 μl per well, mix thoroughly, and incubate in a 37°C incubator for 90 minutes.
[0049] c. Manual washing: Discard the liquid in the wells, fill each well with the washing liquid, let it stand for 5 seconds...
Embodiment 3
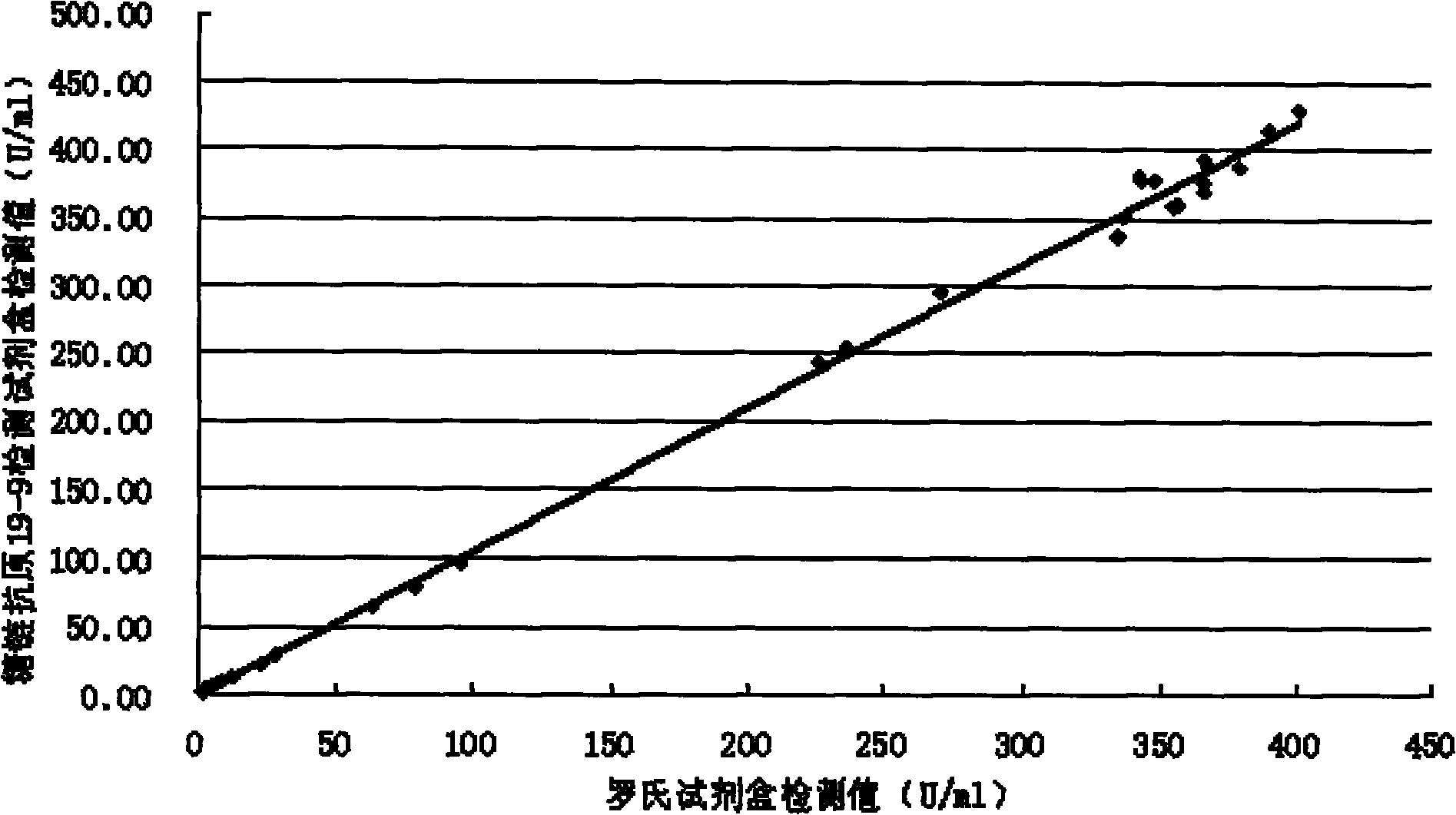
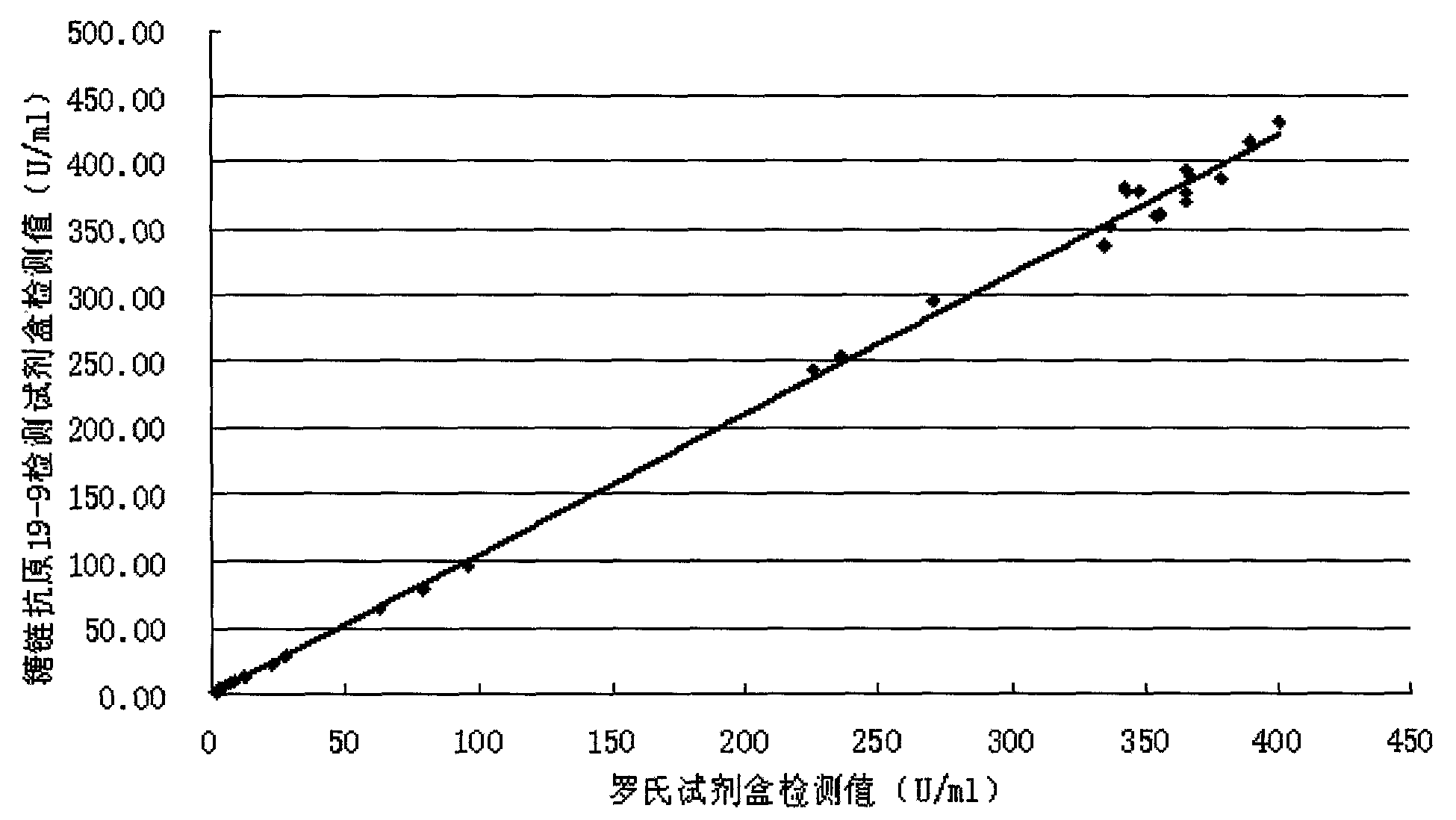
[0058] Methodological Identification of CA19-9 Quantitative Detection Kit
[0059] According to the conventional manufacturing and identification procedures in the art, the test kit prepared in Example 1 is tested and the results are as follows:
[0060] 1. Accuracy
[0061] The known content sample is detected with the kit prepared in Example 1 of the present invention, and the recovery rate is calculated (recovery rate=recovery amount / addition amount×100%), the sample size is not less than 10, and the results are as follows:
[0062] Amount added (U / ml)
[0063] 2. Specificity
[0064] The known content analogue is detected with the kit prepared in the embodiment of the present invention, and the cross-reaction rate is calculated (cross-reaction rate=detection value / addition amount×100%), and the results are as follows:
[0065] name
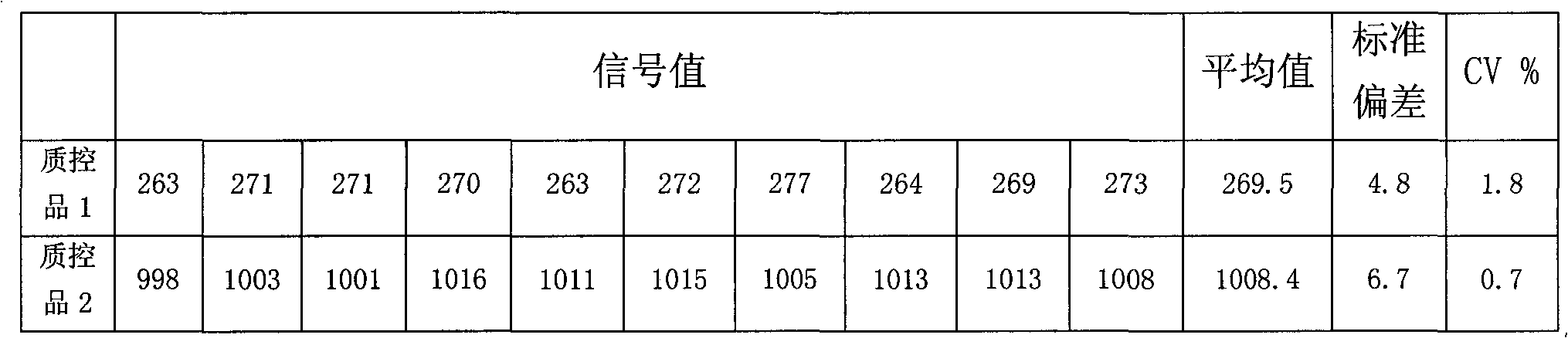
[0066] 3. Precision
[0067] Make 10 parallel wells of quality control product 1 and quality control product 2 respect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com