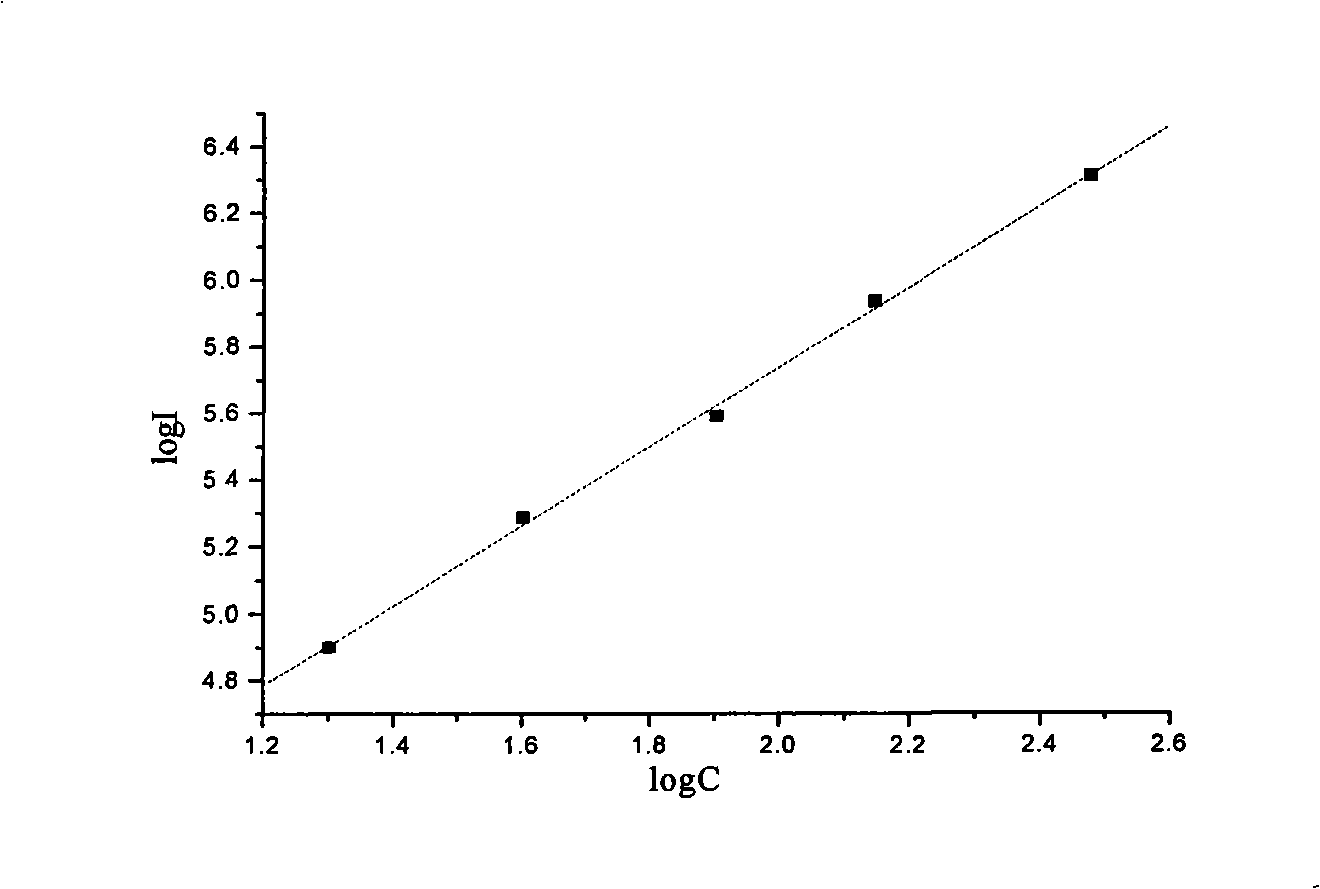
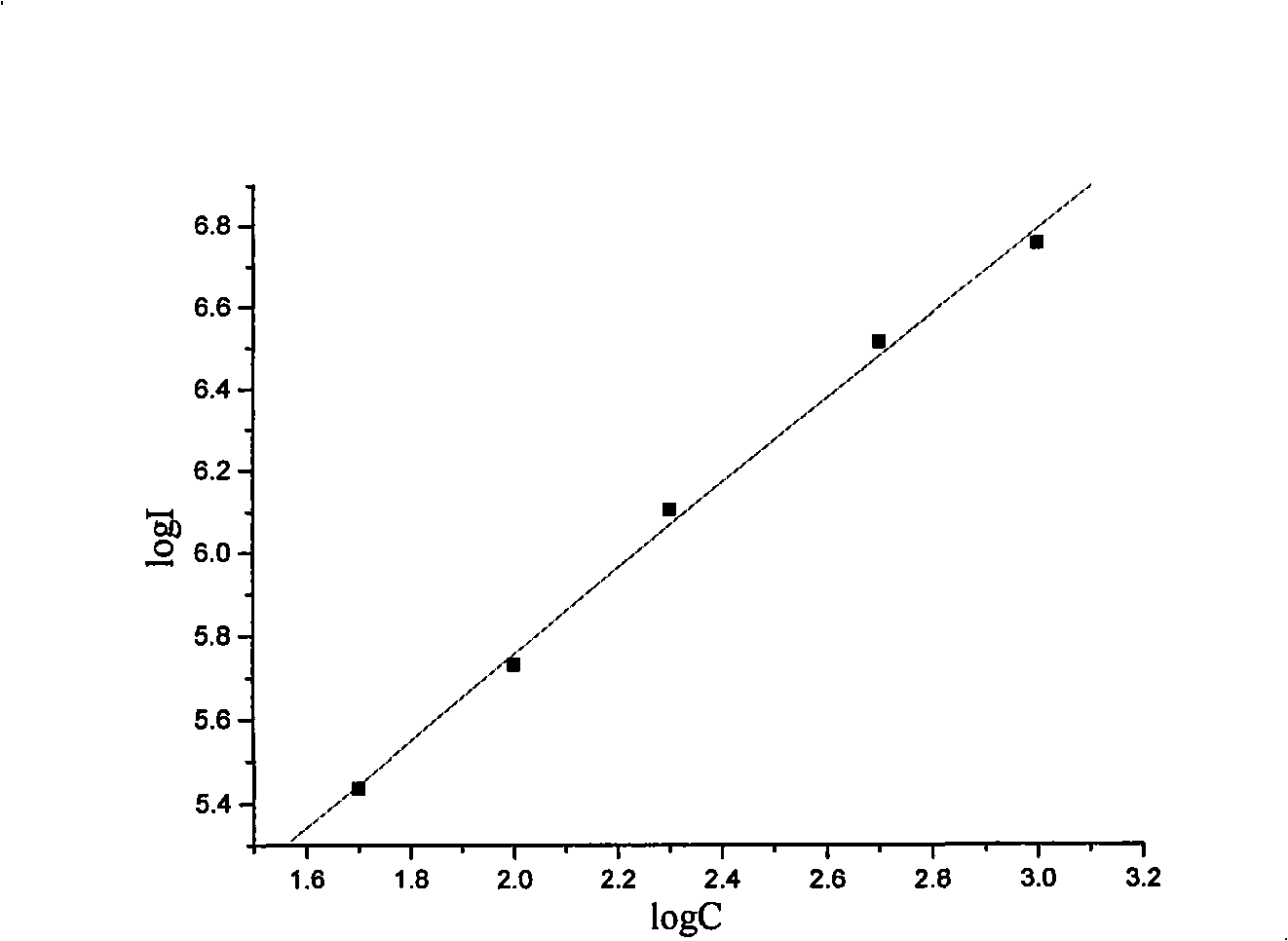
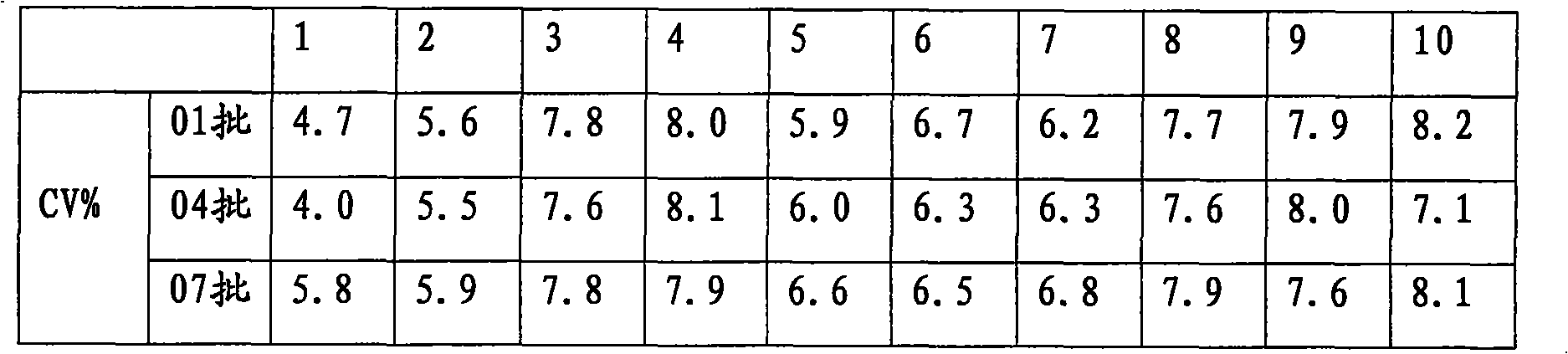
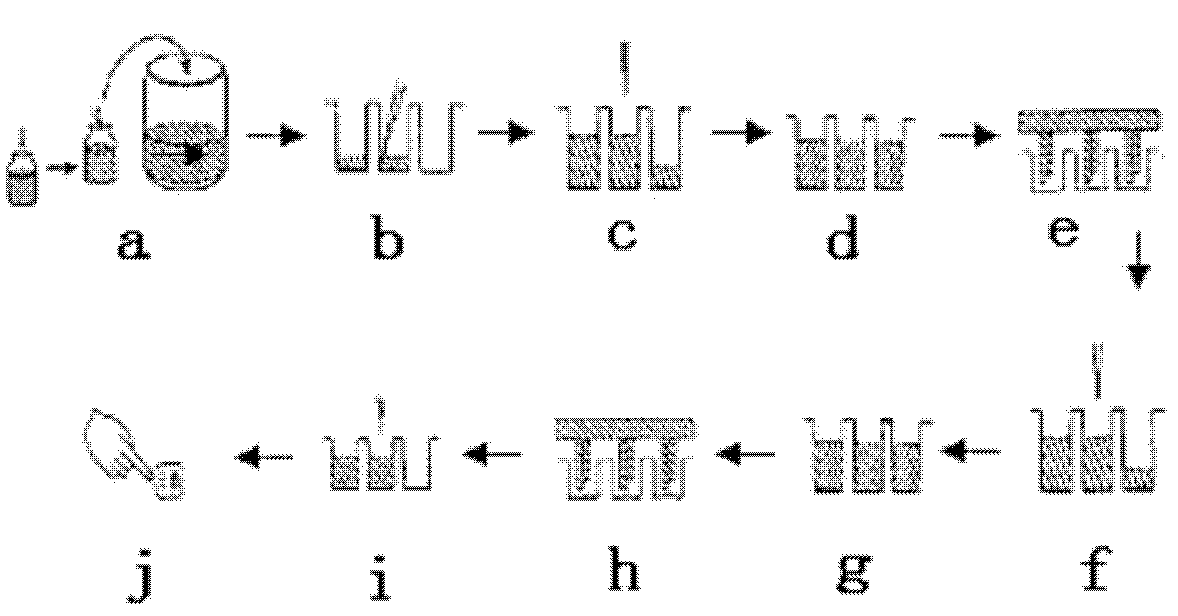
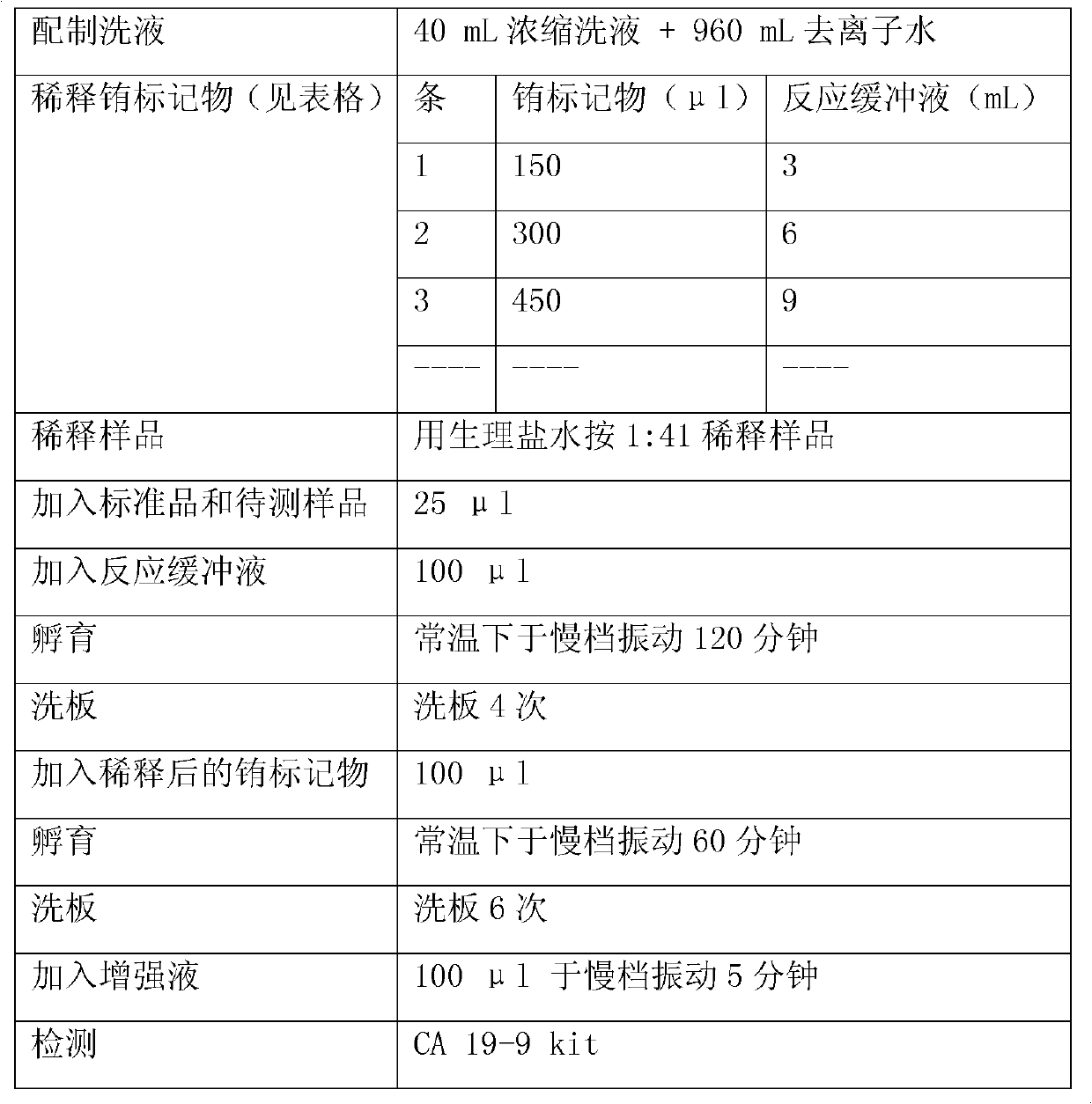
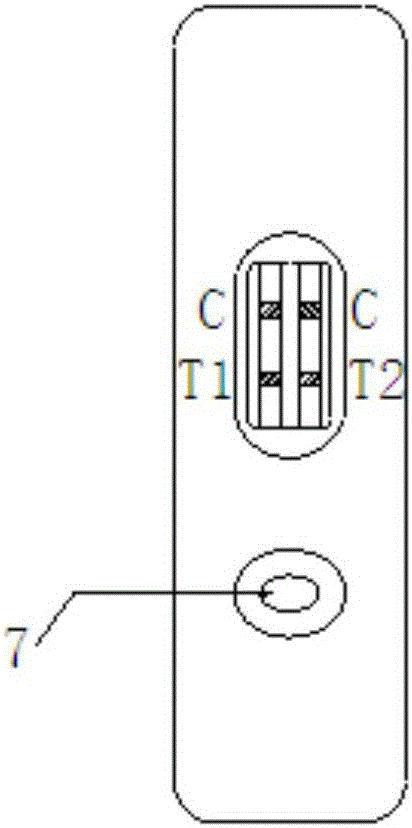
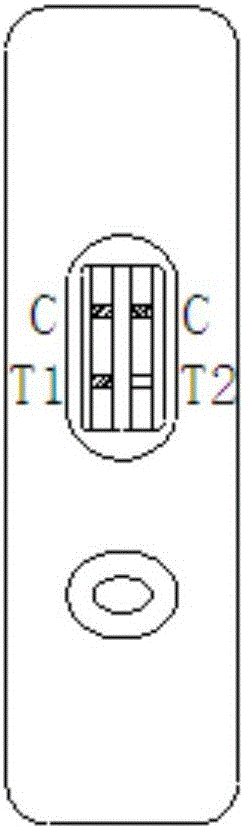
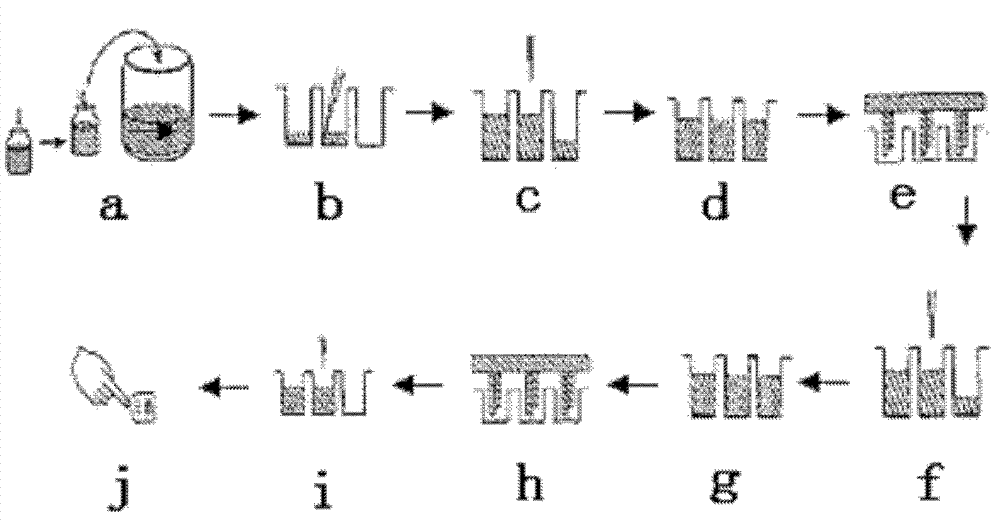
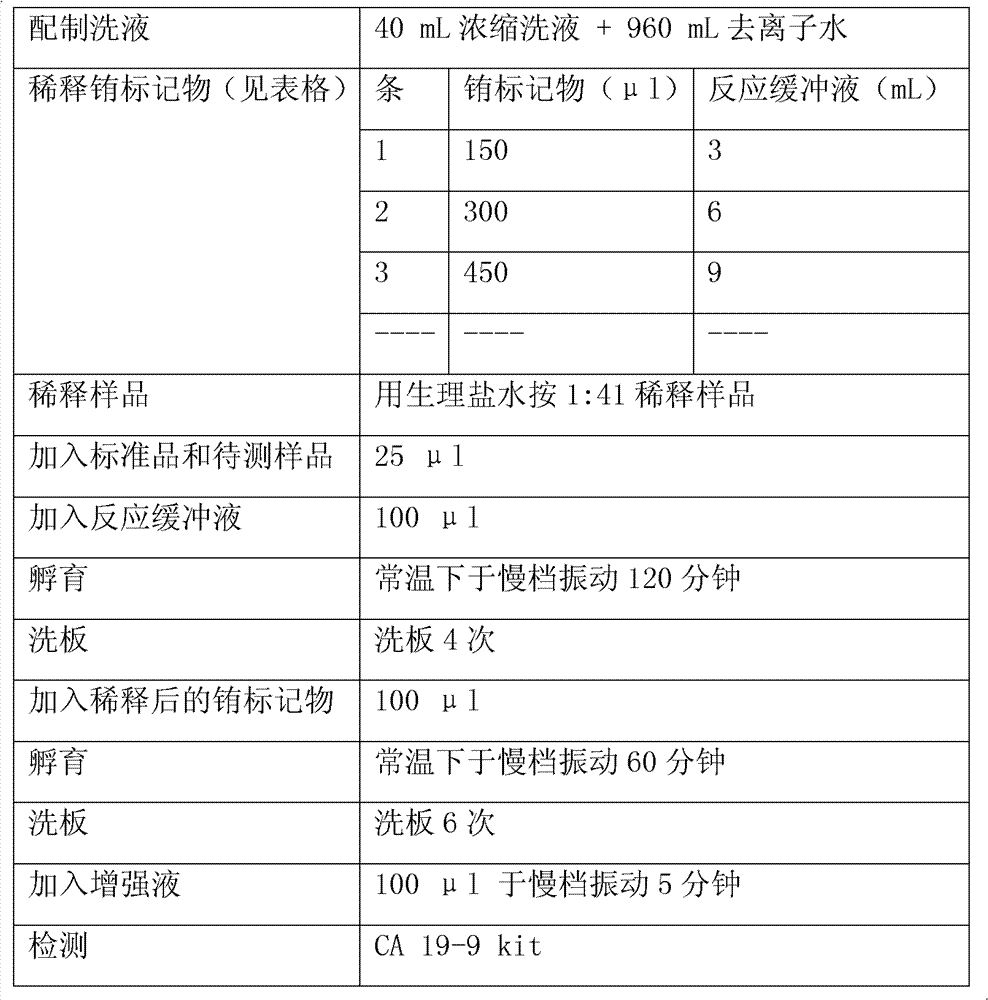
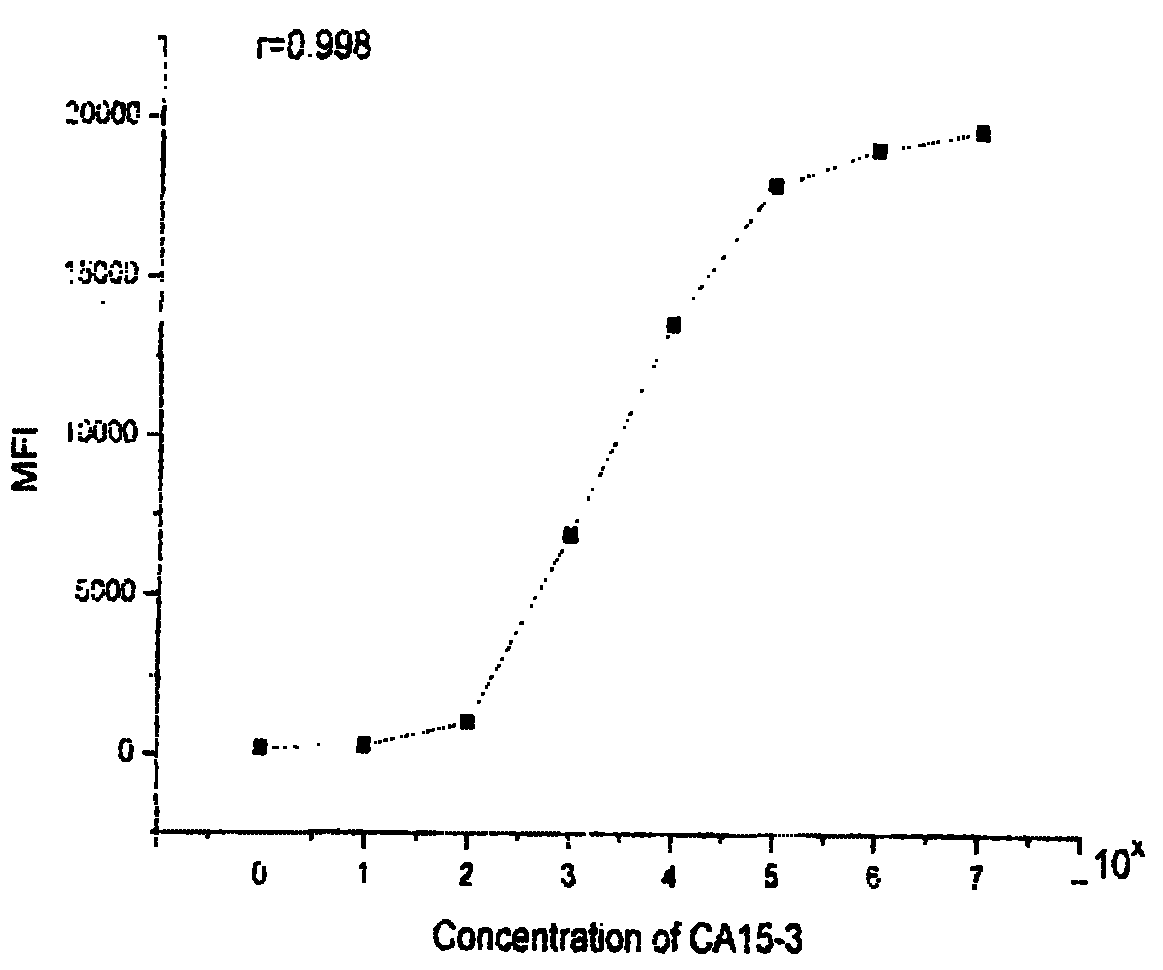
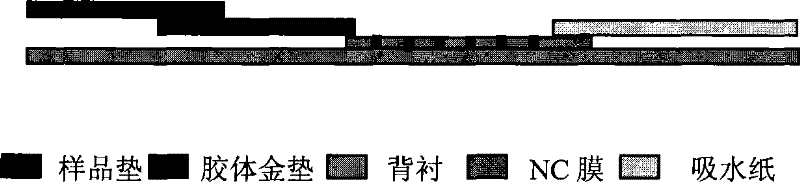
Patents
Literature
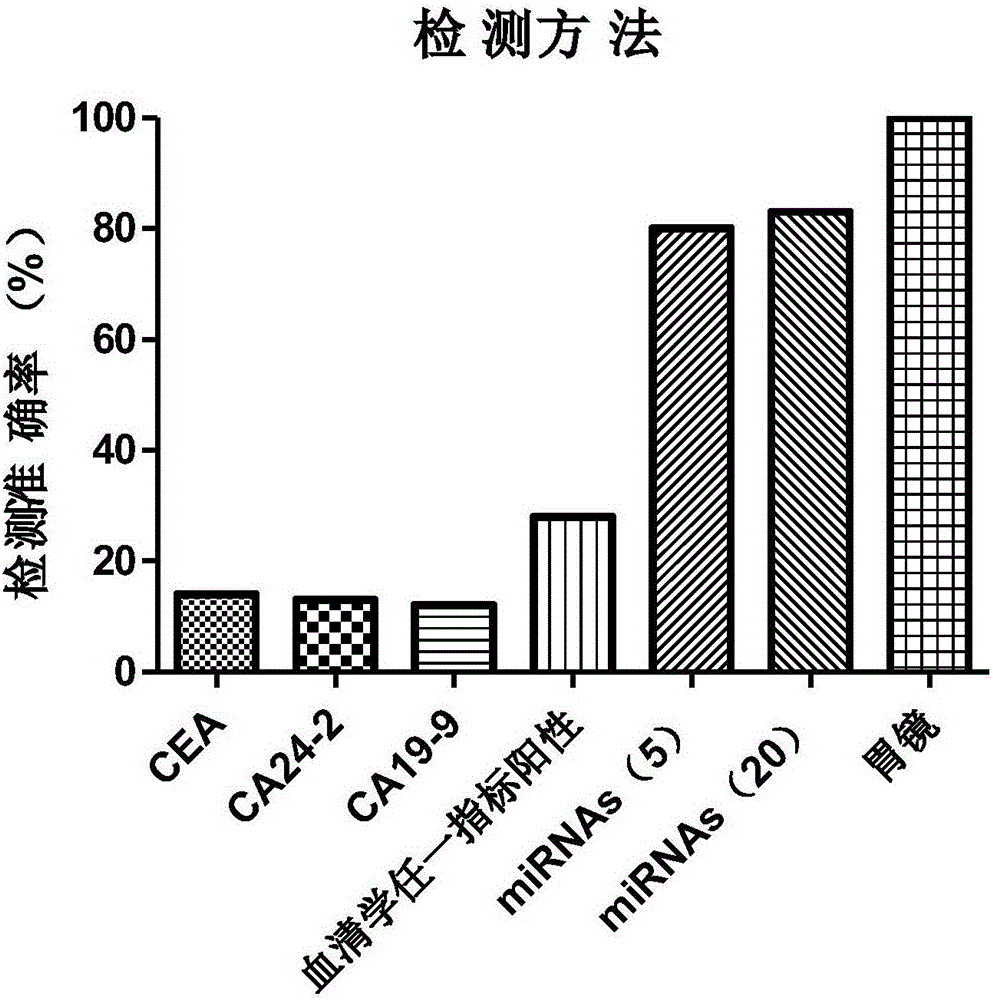
35 results about "CA19-9" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Carbohydrate antigen 19-9 (CA19-9), also known as sialyl-Lewisᴬ, is a tetrasaccharide which is usually attached to O-glycans on the surface of cells. It is known to play a vital role in cell-to-cell recognition processes. It is also a tumor marker used primarily in the management of pancreatic cancer.
Magnetic microparticle chemiluminescence enzyme immune analytic reagent kit for detecting saccharide antigen and its use method
InactiveCN101324579AQuantitative detection of carbohydrate antigen contentLow pre-processing requirementsMaterial analysisCarbohydrate antigenMicroparticle
The invention relates to a magnetic corpuscule chemiluminescent enzyme immunoassay kit for detecting carbohydrate antigen and the application method thereof. The kit comprises FITC antibody-coated magnetic corpuscules; a marker solution prepared by mixing the FITC-marked carbohydrate antigen monoclonal antibody and the enzyme-marked carbohydrate antigen monoclonal antibody; a carbohydrate antigen standard sample solution; a concentrated washing solution; and a luminescent substrate solution, wherein carbohydrate antigen optionally adopts one of CA72-4, CA50, CA19-9, CA242, CA15-3, CA27-29 and CA125. The enzyme-marked antibody and the FITC-marked antibody are the monoclonal antibodies corresponding to the antigens. The FITC antibody coating the magnetic corpuscules adopts a polyclonal antibody or a monoclonal antibody. The marker solution is prepared by mixing an FITC-marked capture antibody working solution and an enzyme-marked antibody pair working solution by the volume ratio of 1:(1-3). Compared with the known kit for mensurating the carbohydrate antigen, the kit has the advantages of high flux, high sensitivity, wide linear range, rapidness, etc., and has a wide application prospect for the clinical inspection, etc.
Owner:TSINGHUA UNIV
Male multi-tumor marker detection protein chip and kit thereof
InactiveCN101603966AChemiluminescene/bioluminescenceBiological testingProtein markersProstate cancer
The invention discloses a male multi-tumor marker detection protein chip and a kit thereof. The chip comprises a substrate, protein markers distributed in an array type and point coatings of contrasts, wherein the substrate is a glass substrate or a film substrate; and the tumor markers and the point coatings of the contrasts are seven protein markers of AFP, CEA, NSE, CYFRA21-1, CA19-9, tPSA and SCC-ag, a positive contrast and a negative contrast which are uniformly distributed and latticed on the substrate. A reaction result of various indexes can be obtained only through one reaction by utilizing the protein chip, and the following tumors can be simultaneously screened: primary liver cancer, prostatic cancer, pancreatic cancer, lung cancer, esophageal cancer, gastric cancer and colorectal cancer. The invention is particularly suitable for the general examination of malignant tumors of male asymptomatic groups and high risk groups.
Owner:上海裕隆生物科技有限公司
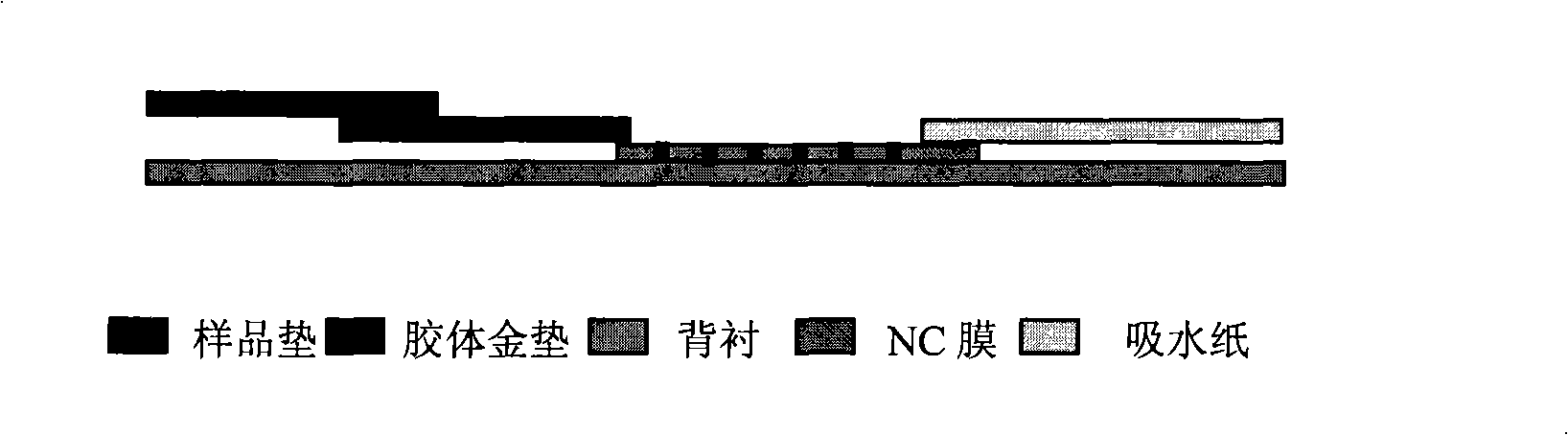
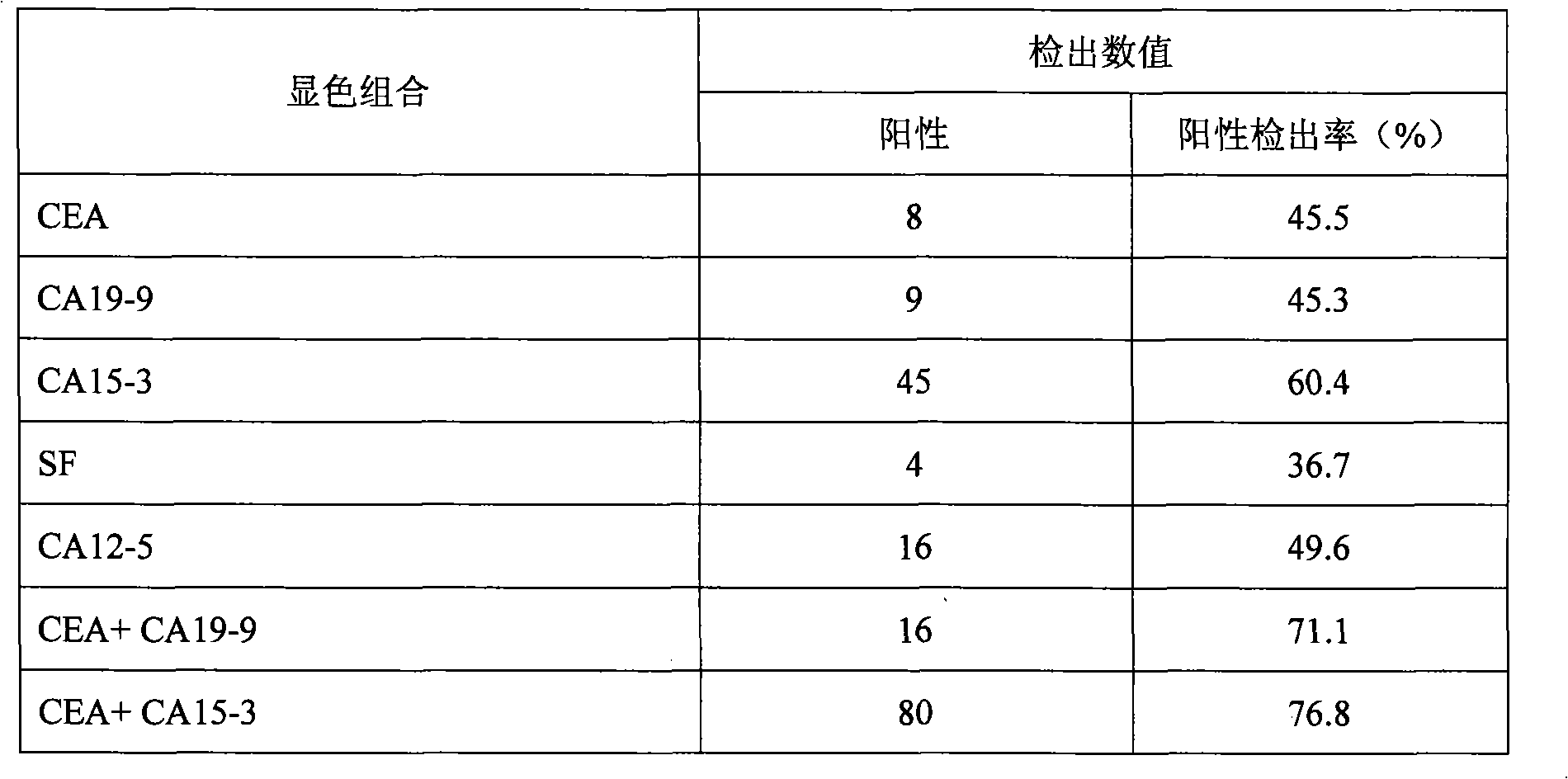
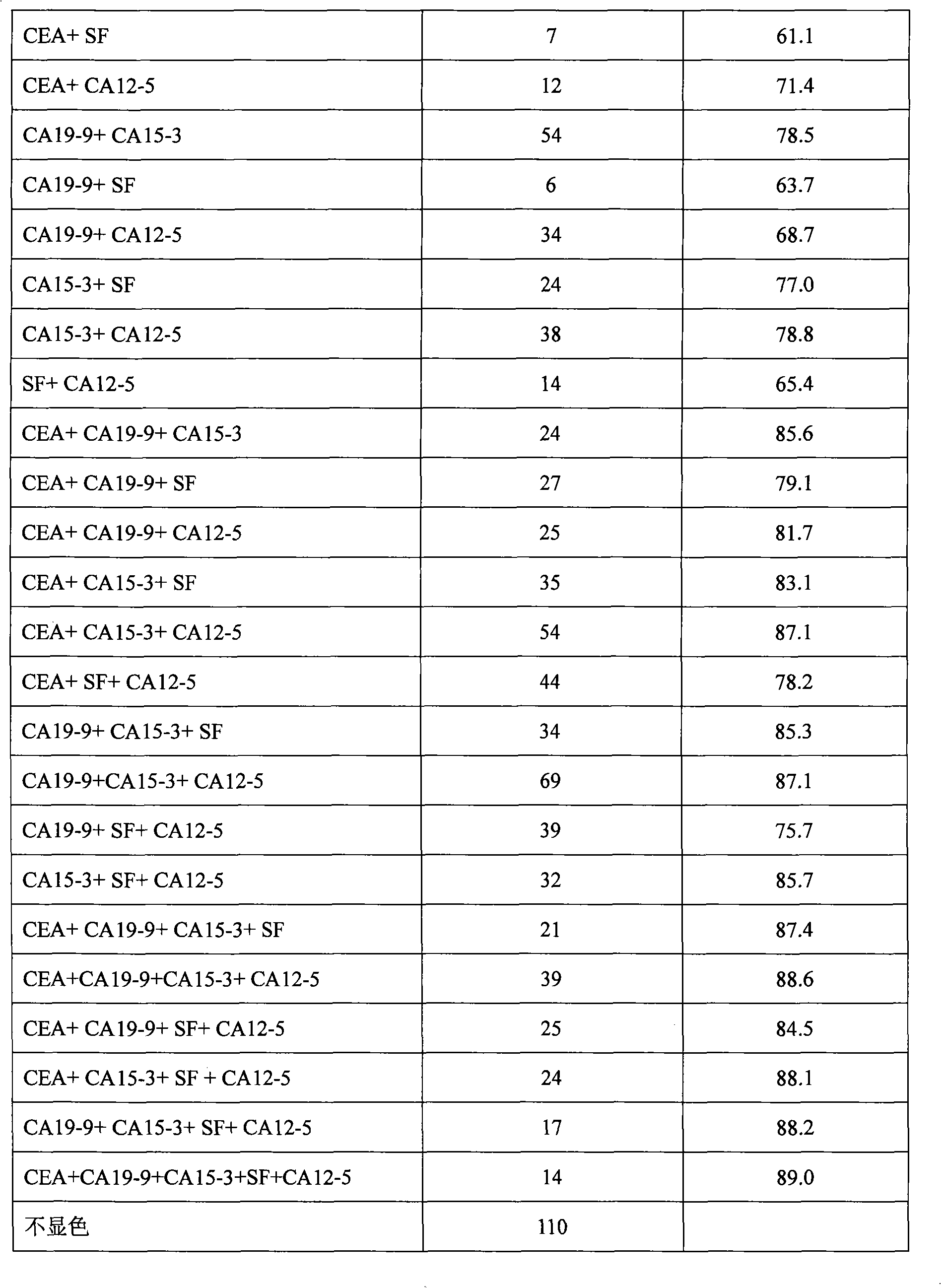
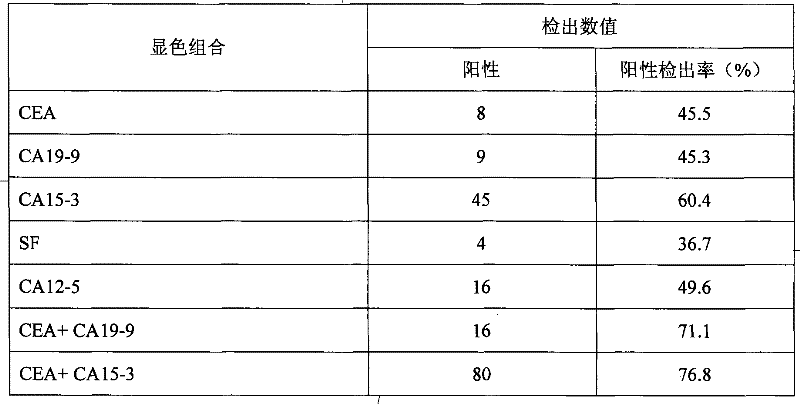
CA15-3, CEA, CA19-9, CA12-5, SF mammary cancer colloidal gold five joint inspection diagnostic reagent kit
The invention discloses a diagnosis kit for breast cancer and preparation method thereof. Five kinds of tumor markers for diagnosis of breast cancer such as CA15-3, CEA, CA19-9, CA12-5, SF are detected together at the same test paper and using immunity chromatography technique the monoclonal antibodies for CA15-3, CEA, CA19-9, CA12-5, SF are fixed on the cellulose nitrate film, at the same time the five kinds of monoclonal antibodies are marked by gold particles of different size and the said gold antibodies are mixed to produce gold affixture pad, finally the gold affixture pad is assembled with a sample treating pad to form a test paper. The said kit quickly test the five kinds of antigens in human whole blood or serum, plasm using double antibody sandwiching method, so as to conquer many defects of previously separation detecting method, only one drop of blood can detect five tumor markers and the detection result is more direct and the sensitivity and specificity of detection is greatly increased.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
Time resolution fluorescence immunochromatography test strip and kit for jointly detecting CA19-9 and Carcino-Embryonic Antigen (CEA)
InactiveCN108132347AWide detection linear rangeQuality improvementBiological testingMicrosphereFluorescence
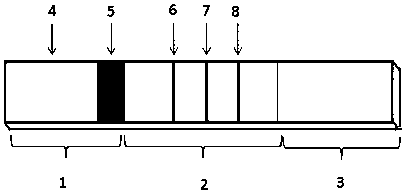
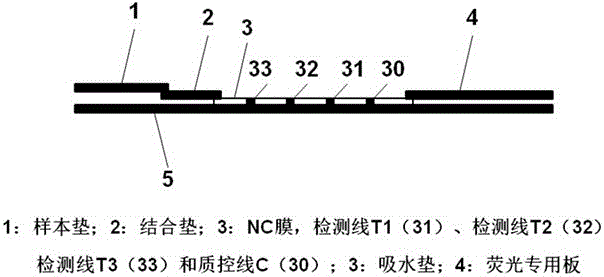
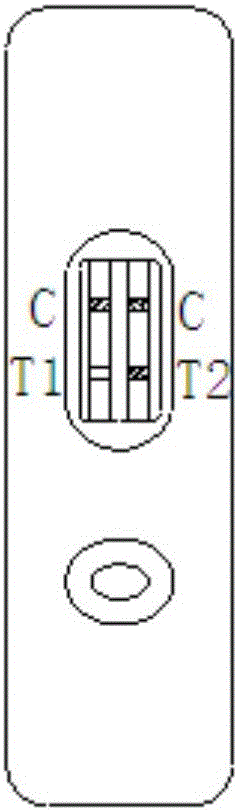
The invention relates to a time resolution fluorescence immunochromatography test strip for jointly detecting CA19-9 and a Carcino-Embryonic Antigen (CEA). The test strip comprises a base plate, a nitrocellulose membrane, a glass fiber cotton and water absorption paper, wherein the nitrocellulose membrane is coated with a CA19-9 monoclonal antibody used as a test 1 wire (T1 wire), a CEA monoclonalantibody used as a test 2 wire (T2 wire) and a sheep anti-mouse polyclonal antibody used as a quality control wire (C wire); the glass fiber cotton is divided into a sample application region and a combination region; the combination region is sprayed with a time resolution fluorescence microsphere-labeled CA19-9 monoclonal antibody compound and a CEA monoclonal antibody compound. According to the time resolution fluorescence immunochromatography test strip and the kit for jointly detecting the CA19-9 and the CEA, test for the concentrations of the CA19-9 and the CEA can be completed within arelatively short time; a test result is high in accuracy and high in sensitivity; a requirement for quick test beside a clinical bed and requirements of a primary community hospital can be met.
Owner:河南省生物工程技术研究中心
Serological marker for detecting pancreatic cancer and a method for using the serological marker
InactiveUS20120295288A1High sensitivityStrong specificityMaterial analysis by electric/magnetic meansIsotope separationULBP2Cancer type
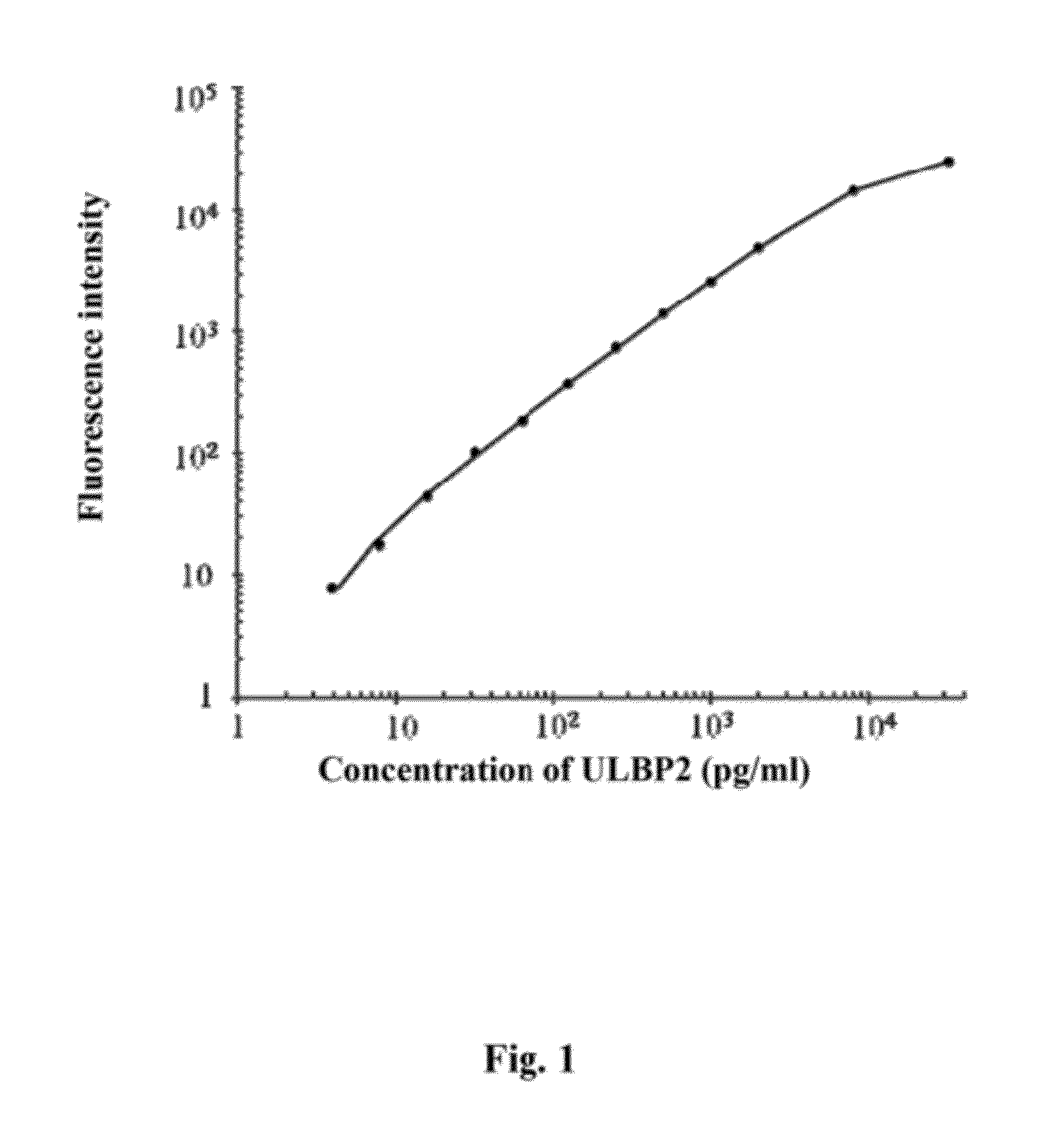
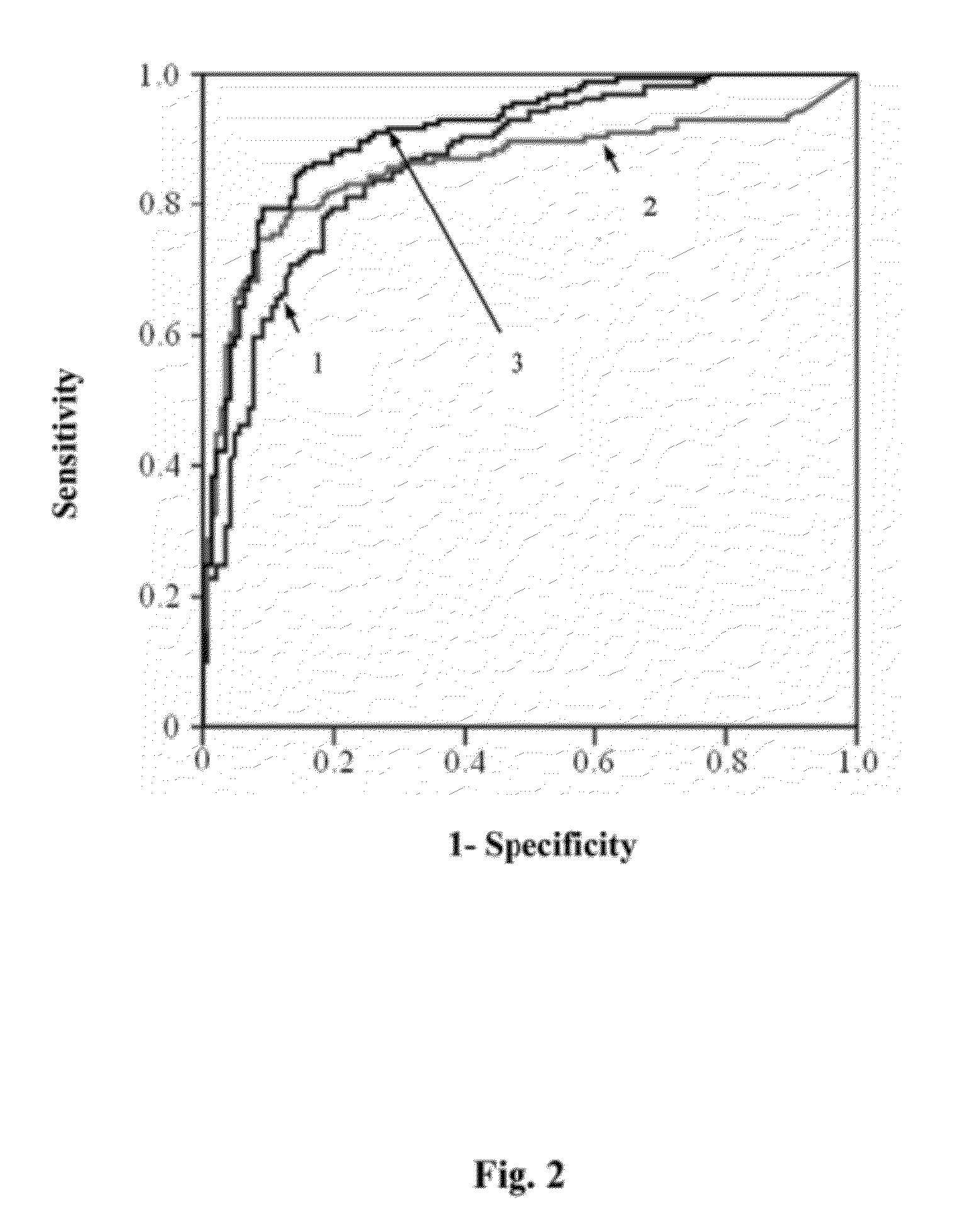
UL16 binding protein 2 (ULBP2) is a protein overexpressed in pancreatic cancer tissues, and the ULBP2 levels are significantly higher in pancreatic cancer patients than those in healthy controls. This invention provides a method to detect pancreatic cancer using ULBP2 as a serological marker. The combination of ULBP2 and CA19-9 promotes the efficacy of pancreatic cancer detection. When measuring the blood ULBP2 levels in patients with other cancer types, including colorectal carcinoma, nasopharyngeal carcinoma and gastric cancer illustrates the blood ULBP2 levels are higher in patients with pancreatic cancer than other cancer types.
Owner:CHANG GUNG UNIVERSITY
Pancreatic ductal adenocarcinoma marker and screening method thereof
ActiveCN109239210AImprove diagnosis rateAccurately reflect differences in metabolic profilesComponent separationPancreas Ductal AdenocarcinomaMetabolite
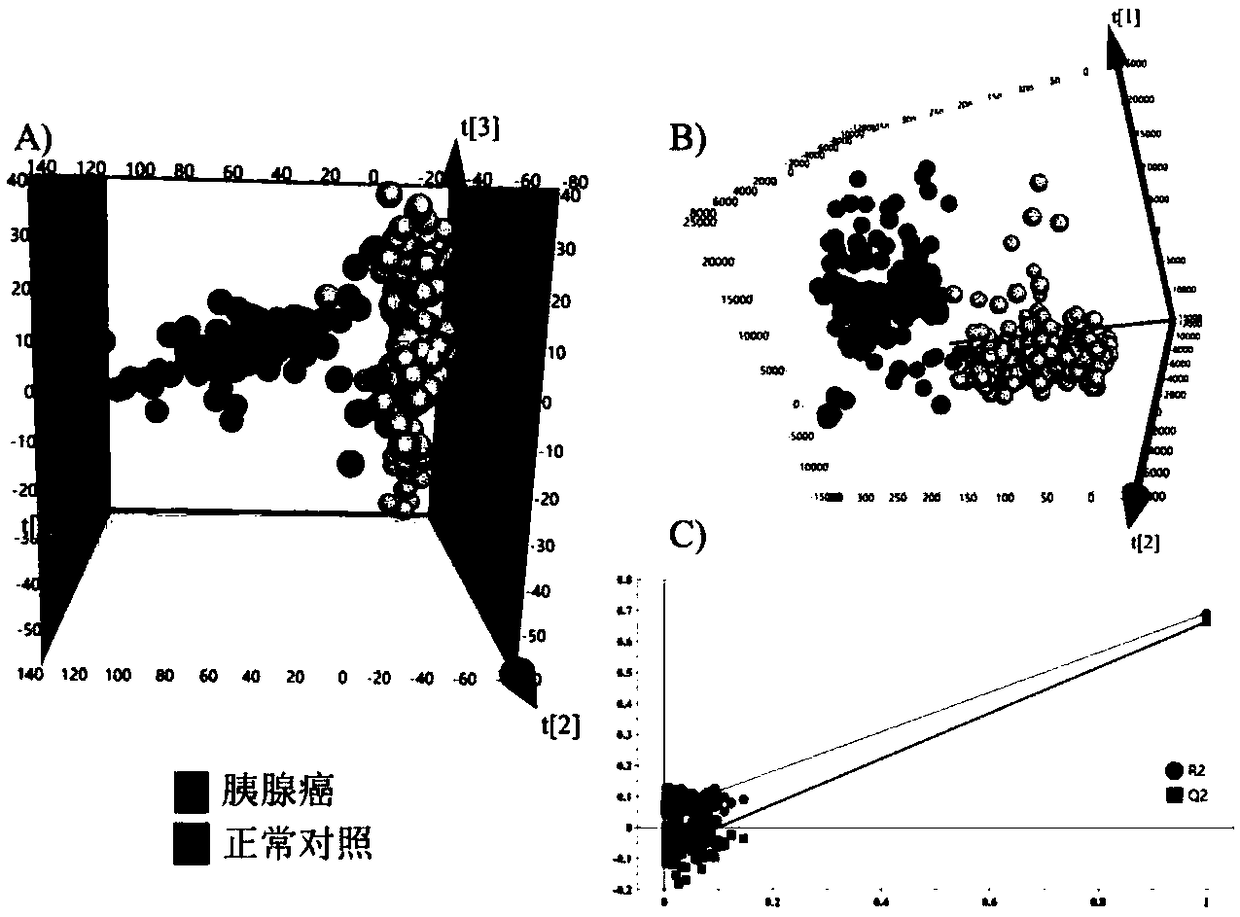
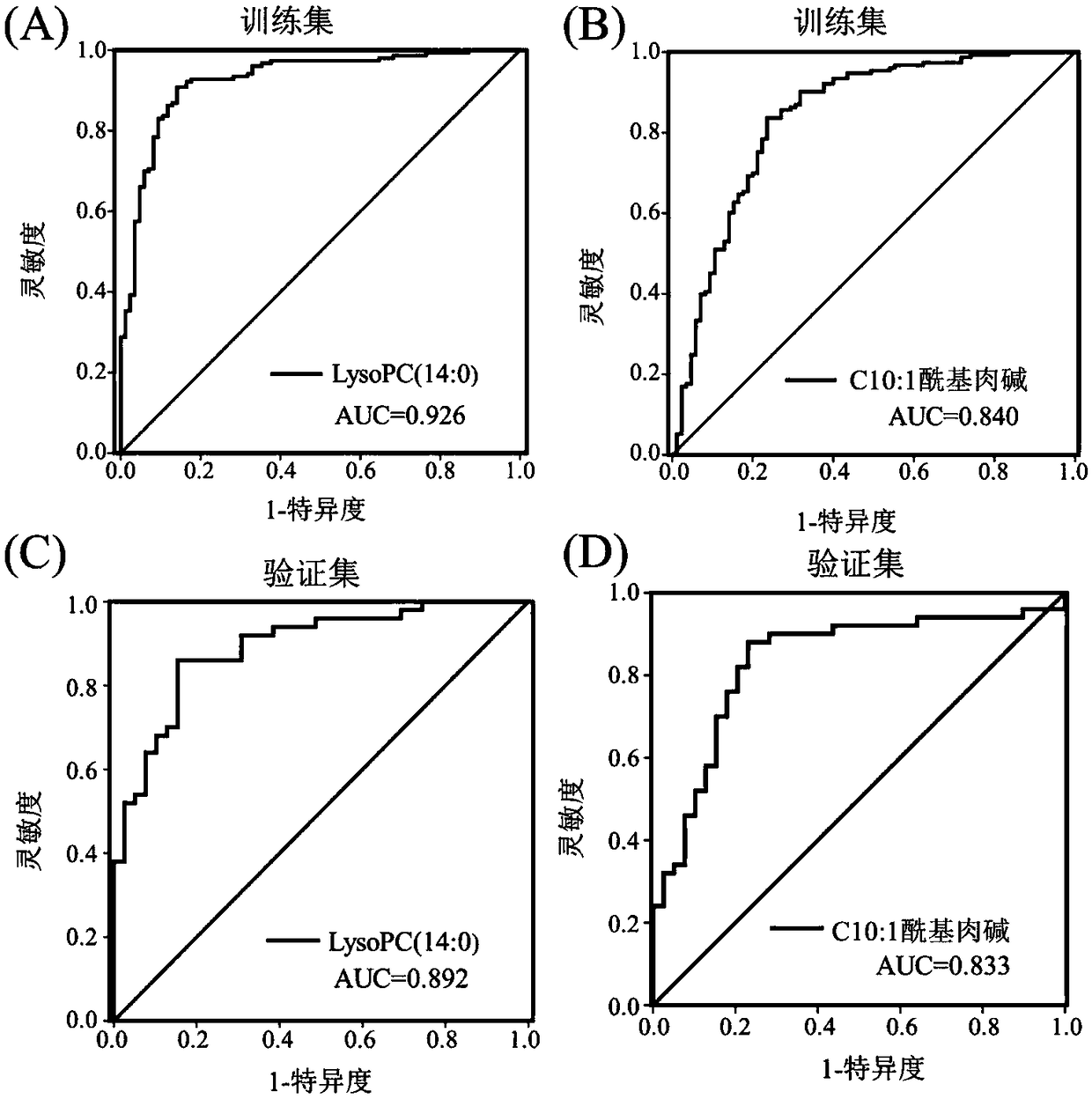
The invention discloses a pancreatic ductal adenocarcinoma marker and a screening method thereof, which belongs to the field of clinical test and diagnosis. In view of the problem that the detection sensitivity and the detection specificity of the existing pancreatic ductal adenocarcinoma diagnosis marker are poor, serum of an early patient of early stage pancreatic duct adenocarcinoma is subjected to a trace metabolomics analysis by the high-performance liquid chromatography-tandem mass spectrometry technology, and different metabolites between normal human and the early stage pancreatic ductal adenocarcinoma patients are found. The different metabolites between normal human and pancreatic ductal adenocarcinoma patients are further analyzed by this technique to find the specific differentmetabolites C10:1 acyl carnitine and lysophosphatidyl choline LysoPC (14:0) of pancreatic ductal adenocarcinoma patients caused by cancer, i.e., the diagnostic molecules of the pancreatic ductal adenocarcinoma. According to the pancreatic ductal adenocarcinoma marker and the screening method thereof, the method can assist CA19-9 in diagnosing pancreatic duct adenocarcinoma patients, and can improve the diagnosis rate for the CA19-9 negative patients by 85%. The method is suitable for the screening of tumor markers.
Owner:HARBIN INST OF TECH
Stomach cancer serological detection and identification kit and method
ActiveCN106755377AExclude false positive resultsAvoid pressure lossMicrobiological testing/measurementAntigenCarbohydrate antigen
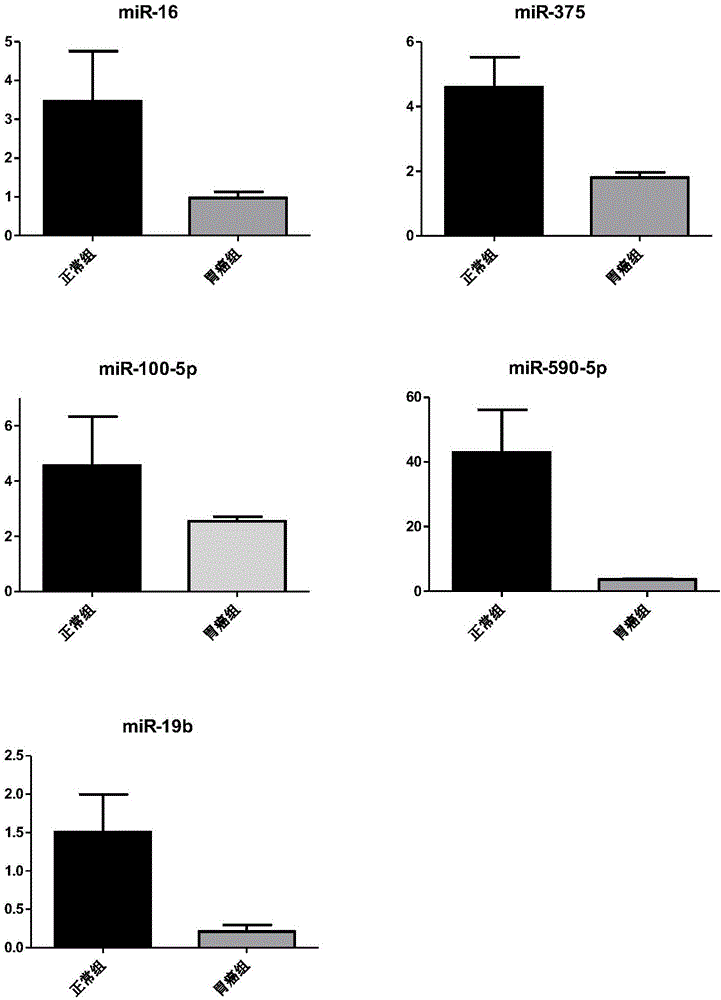
The invention discloses a stomach cancer serological detection and identification kit and a method. The stomach cancer serological detection and identification kit is prepared from a cel-miR-39-5p fragment, a primer, an exosome isolating reagent, an exosome miRNA (Micro Ribonucleic Acid) extracting reagent, a reverse transcription reagent and a real-time fluorescent quantitative PCR (Polymerase Chain Reaction) reagent, wherein the primer comprises a cel-miR-39-5p internal reference primer, an hsa-miR-375 primer, an hsa-miR-590-5p primer, an hsa-miR-19b-3p primer, an hsa-miR-100-5p primer and an hsa-miR-16 primer. When any results of detecting stomach cancer CA (Carbohydrate Antigen)19-9, CA24-2 and CEA (Carcino Embryonie Antigen) of a patient by using serum are positive, and the stomach cancer serological detection and identification kit provided by the invention is then used for carrying out aided detection, false positive results of serological detection can be effectively removed, so that huge mental stress and property loss brought to the patient can be avoided; meanwhile, aiming at the situation that when the results of detecting the stomach cancer CA19-9, CA24-2 and CEA of the patient by using the sera are negative, and the stomach cancer serological detection and identification kit provided by the invention is then used for carrying out the aided detection,, the false negative results of the serological detection can be effectively found, so that the life of the patient can be rescued in time.
Owner:ZHEJIANG PROVINCIAL HOSPITAL OF TRADITIONAL CHINESE MEDICINE
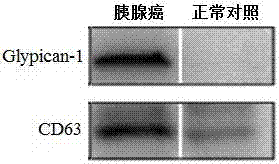
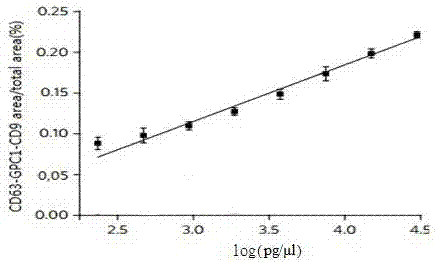
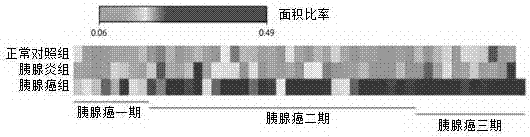
Application of Glypican-1 protein in diagnosis of pancreatic cancer, detection method of positive exosome concentration, and use of detection method
InactiveCN106950374AServe as a diagnosisPlay the role of staging judgment, etc.Raman scatteringBlood plasmaBiology
The invention discloses an application of a Glypican-1 protein in the diagnosis of pancreatic cancer, a detection method of a Glypican-1 positive exosome concentration, and a use of the detection method. A nano-plasma enhanced scattering (nPES) detection technology can be used to quantify exosomes in serum, an exosome extracting process is omitted, the characteristic antigen of the exosomes is used as a target, and the antigen and a corresponding antibody carrying gold nanoparticles form an antigen-antibody complex in order to obtain exosomes, and the amount of the exosomes is reflected by using the light radiation principle of the gold nanoparticles. The Glypican-1 is a pancreatic cancer-derived exosome biomarker, and the content of Glypican-1 positive exosomes in blood plasma has specific specificity even in early pancreatic cancer, so the exosome content index can be used to diagnose the pancreatic cancer, and the exosome level is closely related to the staging and progression of pancreatic cancer patients. Experiments prove that the method using the nPES detection technology to detect the content of the Glypican-1 exosomes in the serum is concise and accurate, and has better sensitivity and specificity than CA19-9 in the diagnosis of the pancreatic cancer.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Colorectal cancer marker galectin, method for analyzing galectin concentration in blood sample, and kit for detecting colorectal cancer marker galectin
InactiveUS20130065258A1High positive rateImprove patient capture rateBiological material analysisPeptide preparation methodsGalectin-3 AntibodyPrognostic prediction
The present invention provides a tumor screening marker that can be actually used in clinical practice to detect colorectal cancer, and a tumor progression marker that can complement CEA or CA19-9. Galectin-1 used as a tumor screening marker or a tumor progression marker for colorectal cancer. Galectin-3 used as a tumor screening marker. Galectin-4 used as a tumor progression marker, a tumor screening marker, or a prognostic prediction marker for colorectal cancer. A method of analyzing the galectin concentration in a collected blood sample using the galectin. A colorectal cancer marker detection kit comprising a detection antibody selected from the group consisting of a fluorescently labeled galectin-1 antibody, a fluorescently labeled galectin-3 antibody, and a fluorescently labeled galectin-4 antibody.
Owner:SHIMADZU CORP +1
Kit for dry-type fluorescent quantum dot joint detection diagnosis of pancreatic cancer
The invention discloses a kit for dry-type fluorescent quantum dot joint detection diagnosis of pancreatic cancer and a preparation method of the kit. The kit can be used for joint detection of three tumor markers Ca19-9, CEA, CA242 for clinical diagnosis of pancreatic cancer on the same detection strip, monoclonal antibodies of CA19-9, CEA and CA242 are fixed on a nitrocellulose film by means of an immunochromatography technique for preparation of a reaction film, the three monoclonal antibodies are marked with quantum dots emitting different light, the antibodies marked with the quantum dots are mixed and sprayed on a glass fiber film for preparation of a conjugate pad, and the conjugate pad, a sample pad and a water absorbing pad are combined to form a quantum dot immunostrip. The kit is prepared with a double-antibody sandwich method, three antigens in whole blood or serum and plasma are quickly detected, numerous defects of existing independent detection methods are avoided, the three tumor markers can be detected simultaneously with one drop of blood, the result is more direct, the sensitivity and the specificity are both remarkably improved, and accordingly, the kit has great potential in clinical application.
Owner:重庆高圣生物医药有限责任公司
Marker for detecting pancreatic cancer
InactiveCN104395758AJudge possibilityPrognostic status can be monitoredTumor rejection antigen precursorsTumor specific antigensPancreatitisGlycoprotein
Owner:NITTO BOSEIKI CO LTD
Colloidal gold immunochromatographic test strip for aided detection of pancreatic cancer and preparation method of golloidal gold immunochromatographic test strip
InactiveCN105785013AEasy to operateHigh sensitivityBiological material analysisBiological testingMonoclonal antibodyAbsorbent Pads
The invention provides a colloidal gold immunochromatographic test strip for aided detection of pancreatic cancer and a preparation method of the golloidal gold immunochromatographic test strip. The strip comprises a bottom plate, a sample absorbent pad, a colloidal gold conjugate pad, a nitrocellulose membrane and a water absorption pad; when the strip is prepared, the sample absorbent pad, the colloidal gold conjugate pad, the nitrocellulose membrane and the water absorption pad are sequentially adhered to the bottom plate in an overlapped manner; the colloidal gold conjugate pad is coated with colloidal gold-labeled CA19-9 monoclonal antibody and colloidal gold-labeled monoclonal antibody of helicobacter pylori hemagglutinin antigen; the nitrocellulose membrane is coated with a detection line 1, a detection line 2 and a quality control line; the detection line 1 is coated with colloidal gold-labeled CA19-9 monoclonal antibody; the detection line 2 1 is coated with colloidal gold-labeled monoclonal antibody of helicobacter pylori hemagglutinin antigen; the quality control line is coated with goat anti-rabbit IgG antibody. The strip is simple in preparation and operation steps and high in sensitivity, and provides a good basis for early examination of pancreatic cancer.
Owner:卢连伟
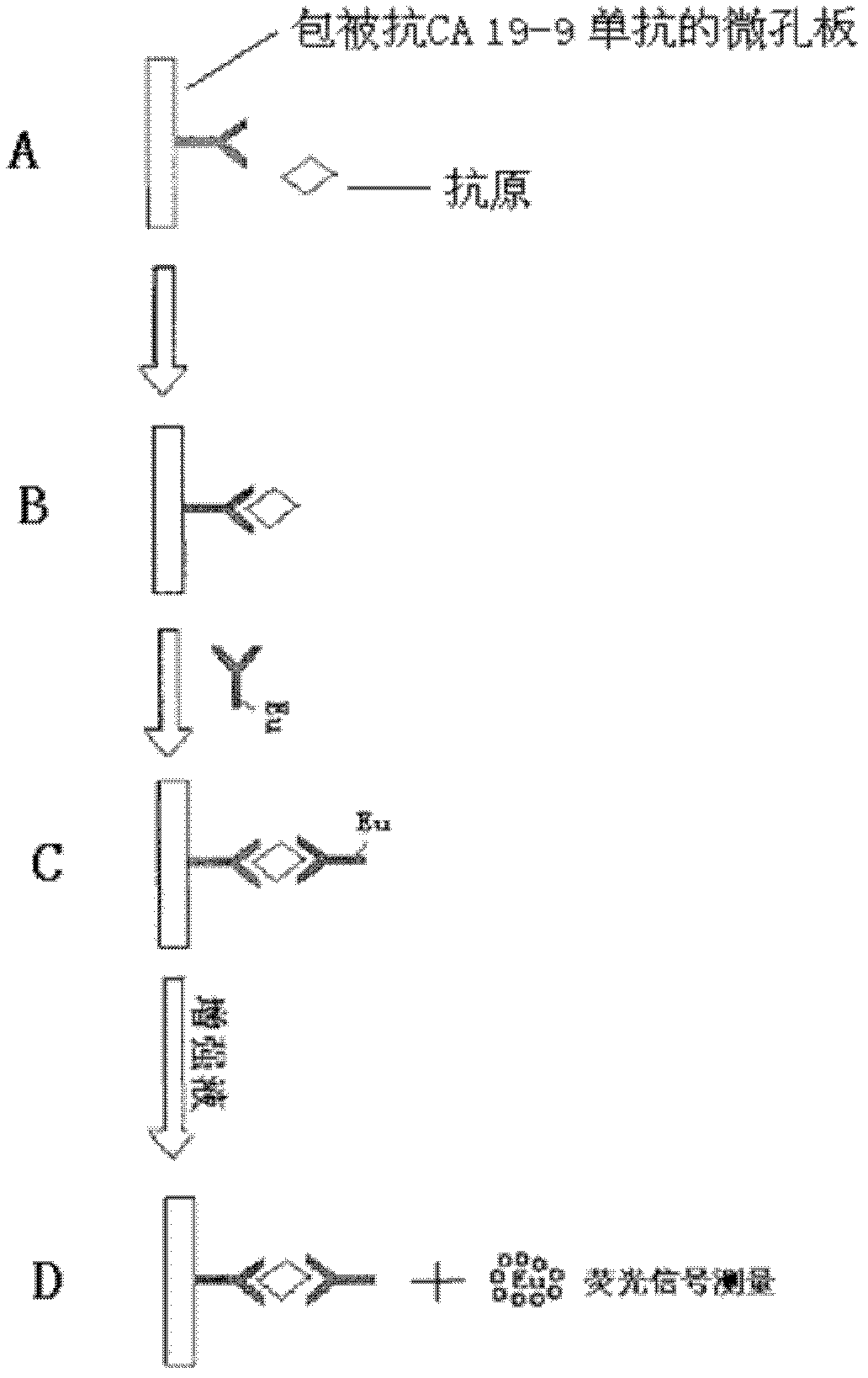
Carbohydrate antigen CA19-9 assay kit and detection method using the same
InactiveCN107389946ALong validity periodHigh Luminescence Quantum YieldBiological testingCarbohydrate antigenMagnetic bead
The invention discloses a carbohydrate antigen CA19-9 assay kit. The kit comprises a magnetic separation reagent R1, a labeling reagent R2, a CA19-9 calibration product and a CA19-9 quality control product. The invention also provides a preparation method of the kit. The magnetic separation reagent R1 in the kit is prepared through coupling a CA19-9 monoclonal antibody to the surface of a carboxyl magnetic bead. The labeling reagent R2 is prepared through coupling a luminescent substance acridinium ester to a paired CA19-9 monoclonal antibody. The reactions occur under nearly homogeneous conditions. Through use of an excitation solution, the reaction product antibody-antigen-antibody sandwich complex can immediately release photons at 430 nm and can release strong photons without use of a luminescent enhancer and a catalyst so that luminescence detection can be directly carried out. Through optimizing the experimental processes of magnetic bead coating with an antibody and antibody labeling with acridinium ester, the stability and the detection sensitivity of the kit are greatly improved. The kit has a wide linear detection range, is easy to operate and has a fast detection rate.
Owner:北京健安生物科技有限公司
Early diagnosis method and diagnostic kit for liver cancer
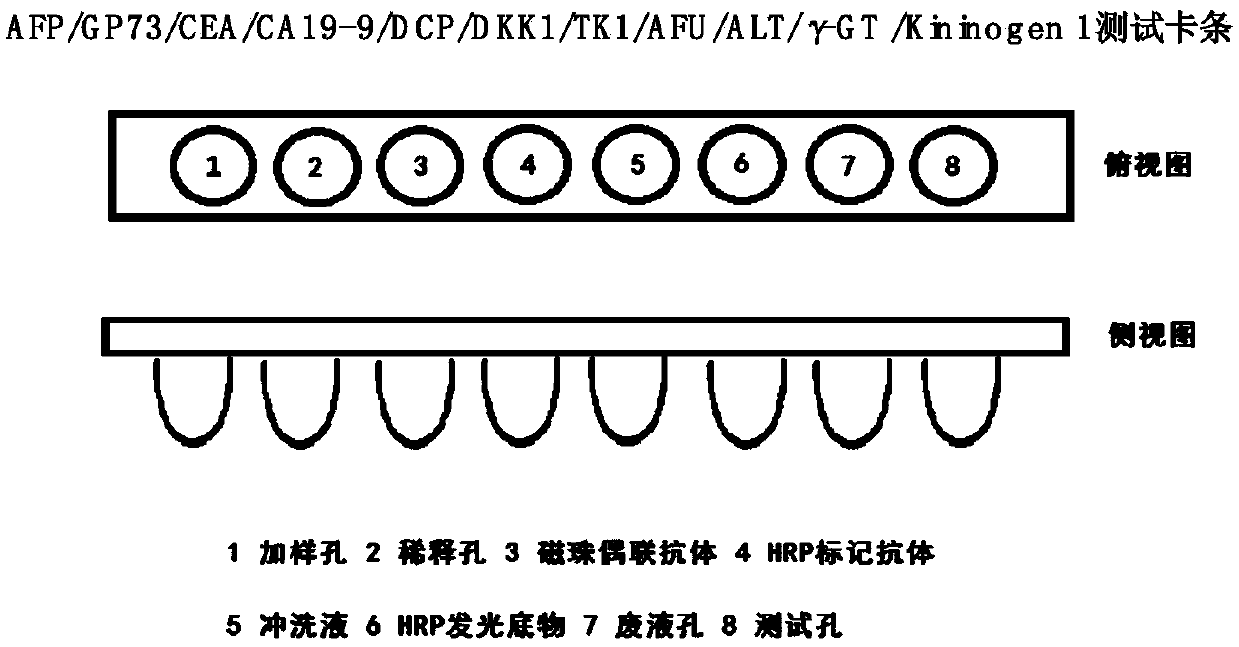
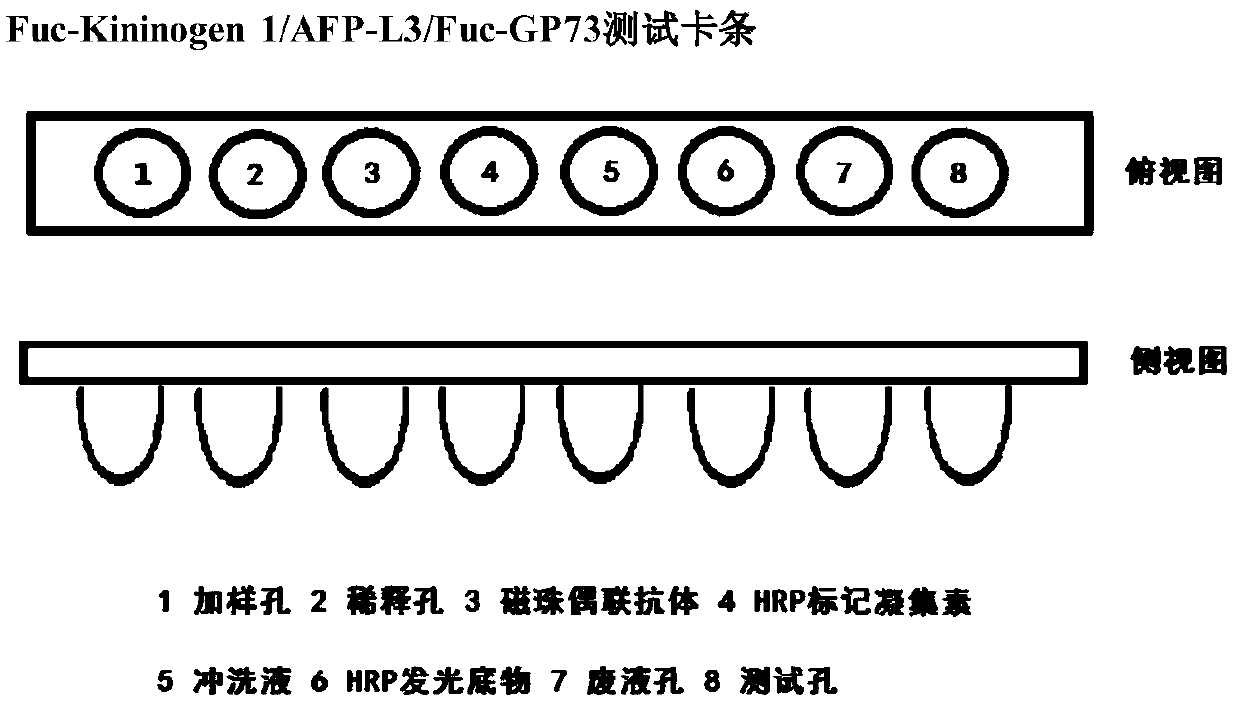
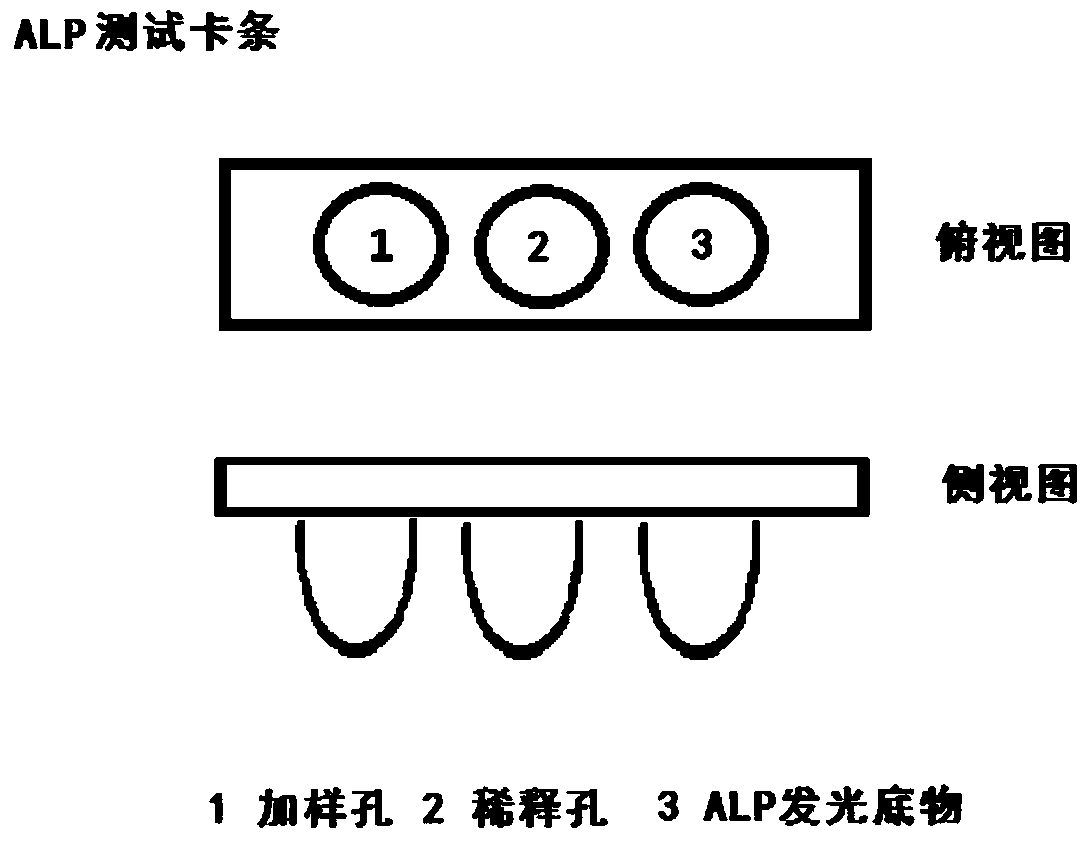
The invention provides an early diagnosis method of liver cancer for a subject, comprising the steps of: 1) obtaining a biological sample from the subject; 2) detecting the content of a plurality of biomarkers in the biological sample, wherein the plurality of biomarkers are selected from two or more of AFP, AFP-L3, GP73, Fuc-GP73, DCP, CEA, CA19-9, TK1, DKK1, [Gamma]-GT, ALP, AFU, ALT and Fuc-Kininogen 1; 3) calculating the probability that the subject has liver cancer based on the detection result of step 2); and 4) comparing the probability with a set threshold, and when the probability isgreater than or equal to the set threshold, the subject is considered to have liver cancer; when the probability is less than the set threshold, the subject is considered not to have liver cancer. Theinvention also provides a corresponding detection kit and a detection device. The early diagnosis method and the diagnostic kit of liver cancer improve the sensitivity and the specificity of early diagnosis of liver cancer by the combined detection of various markers, and provide a more reliable basis for clinical diagnosis of liver cancer.
Owner:BEIJING EXELLON MEDICAL TECH CO LTD
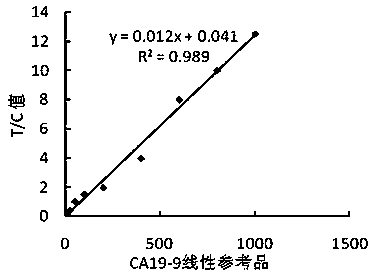
Time resolution immunofluorescence analyzing method and kit for saccharides antigen 19-9
ActiveCN103308683AImprove stabilityHigh analytical sensitivityMaterial analysisAntigenImmunofluorescence
The invention discloses a time resolution immunofluorescence analyzing method and a kit for saccharides antigen 19-9. The analyzing method comprises the following steps of: 1, preparing a solid-phase antibody; 2, preparing a lanthanide ion labeled antibody; and 3, adding a standard product or a sample to be measured of the saccharides antigen 19-9 into a solid-phase material with a solid-phase antibody prepared in the step 1, performing reaction on a buffer liquid, and after the solid-phase material is incubated and washed, adding the diluted labeled antibody and incubating again to perform fluorescence detection. The invention also provides the corresponding kit. The method and the kit have the characteristics of high sensitivity, high specificity and high stability, short detection time and high coincidence rate of detecting national quality control products of CA19-9.
Owner:瑞孚迪生物医学(上海)有限公司
Pancreas cancer duplex detection kit
InactiveCN106053824ASimple and fast operationRapid responseBiological testingCellulosePancreas Cancers
The invention relates to a pancreas cancer duplex detection kit. The detection kit comprises (a) a CA19-9 test strip, which comprises a CA19-9 gold pad of a mouse-anti-human CA19-9 antibody containing a color developing marker, a detection line containing a mouse-anti-human CA19-9 antibody, and a cellulose nitrate membrane containing a goat-anti-mouse IgG quality control line; (b) a mesothelin test strip, which comprises a mesothelin gold pad of a mouse-anti-human mesothelin antibody containing a color developing marker, a detection line containing a mouse-anti-human mesothelin antibody, and a cellulose nitrate membrane containing a goat-anti-mouse IgG quality control line. The provided duplex detection kit has the advantages of convenient operation, quick reaction, high sensitivity, strong specificity, suitability for onsite detection, economy, and practicality.
Owner:上海埃文生物科技有限公司
Magnetic immuno-chromatographic test paper strip for quantitatively detecting tumor associated antigen 19-9 in blood and preparation method thereof
InactiveCN101762698ARealize single-serving wide-range quantitative detectionClinically convenientMaterial analysisMedicineBiotin-binding proteins
The invention relates to a magnetic immuno-chromatographic test paper strip for quantitatively detecting tumor associated antigen 19-9 in blood and a preparation method thereof. A coating film, a magnet particle pad combined with CA19-9 antibodies, a sample pad and a water absorbing pad are sequentially and alternately stuck on a base plate in a staggering way at intervals of 2mm, and then the upper layer is covered by a transparent plastic sealing film to form a test paper strip. A CA19-9 antibody detection line and a quality control line are pre-coated on the coating film. The invention introduces the magnetic immuno-chromatographic technology and the biotin-avidin system into the quantitative detection of CA19-9 in blood, and has the advantages that the detection sensitivity and the reliability are greatly improved, the detection is accurate and rapid, long-time waiting is not required, the cost is low, the operation method is simple and convenient and the detection can be controlled easily.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Marker for detecting pancreatic cancer
InactiveUS20150104816A1Specific determinationTumor rejection antigen precursorsTumor specific antigensPancreatitisGlycoprotein
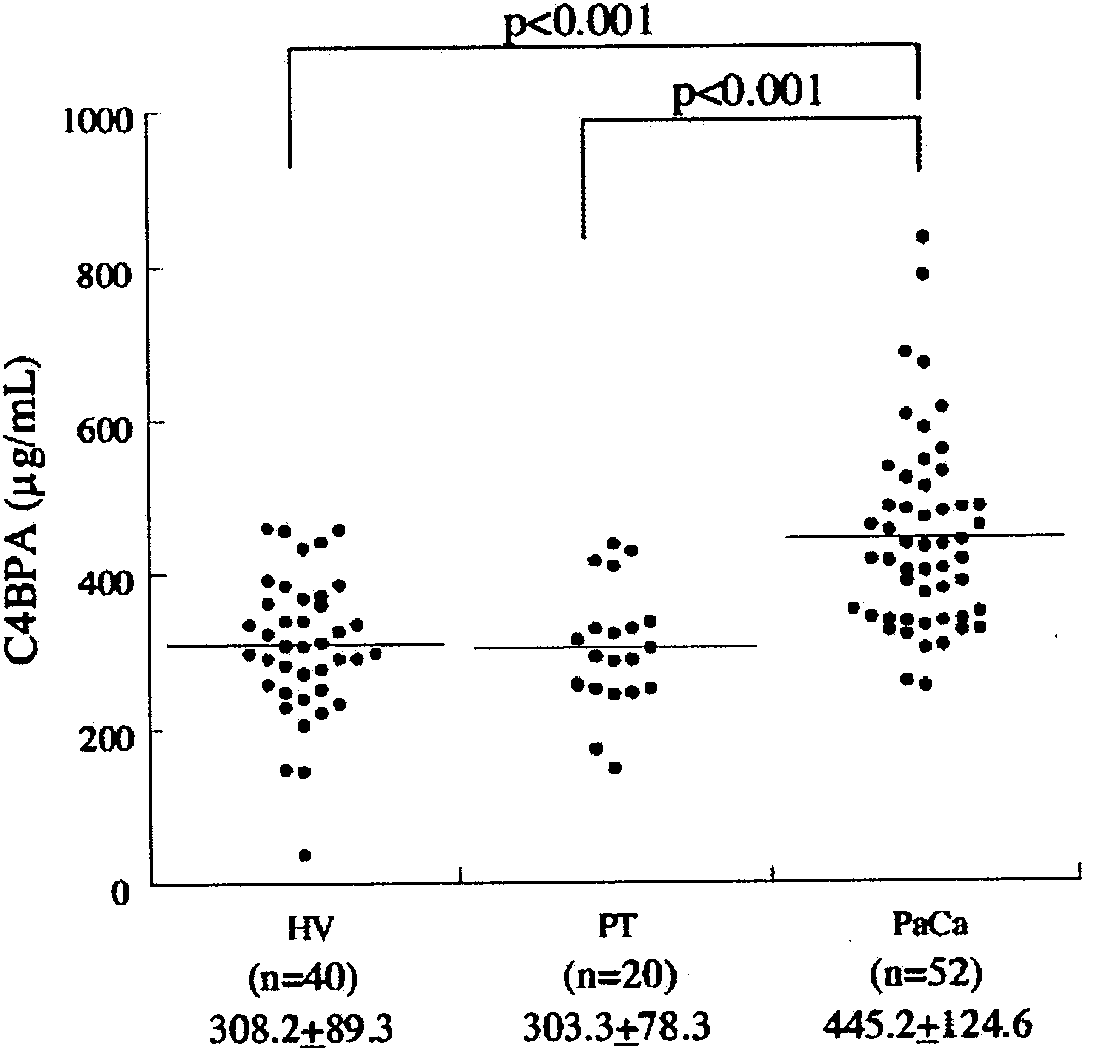
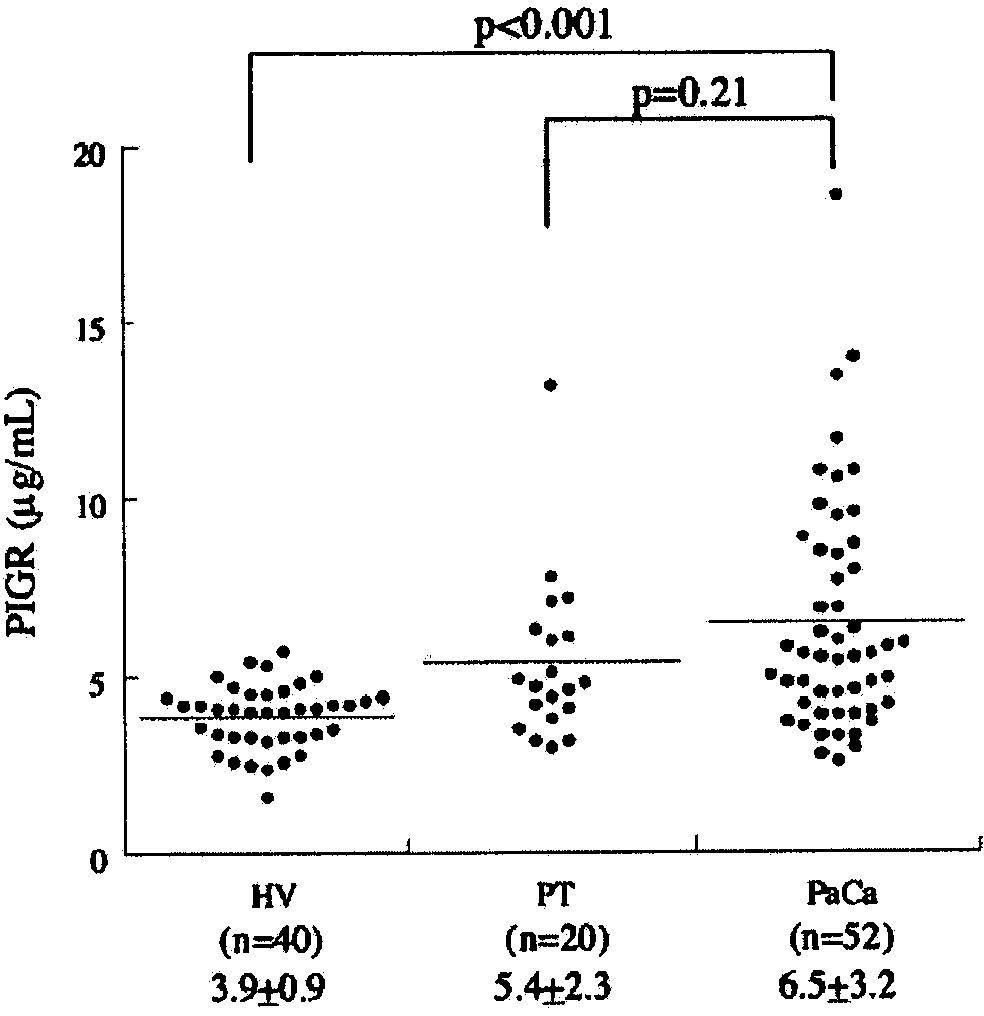
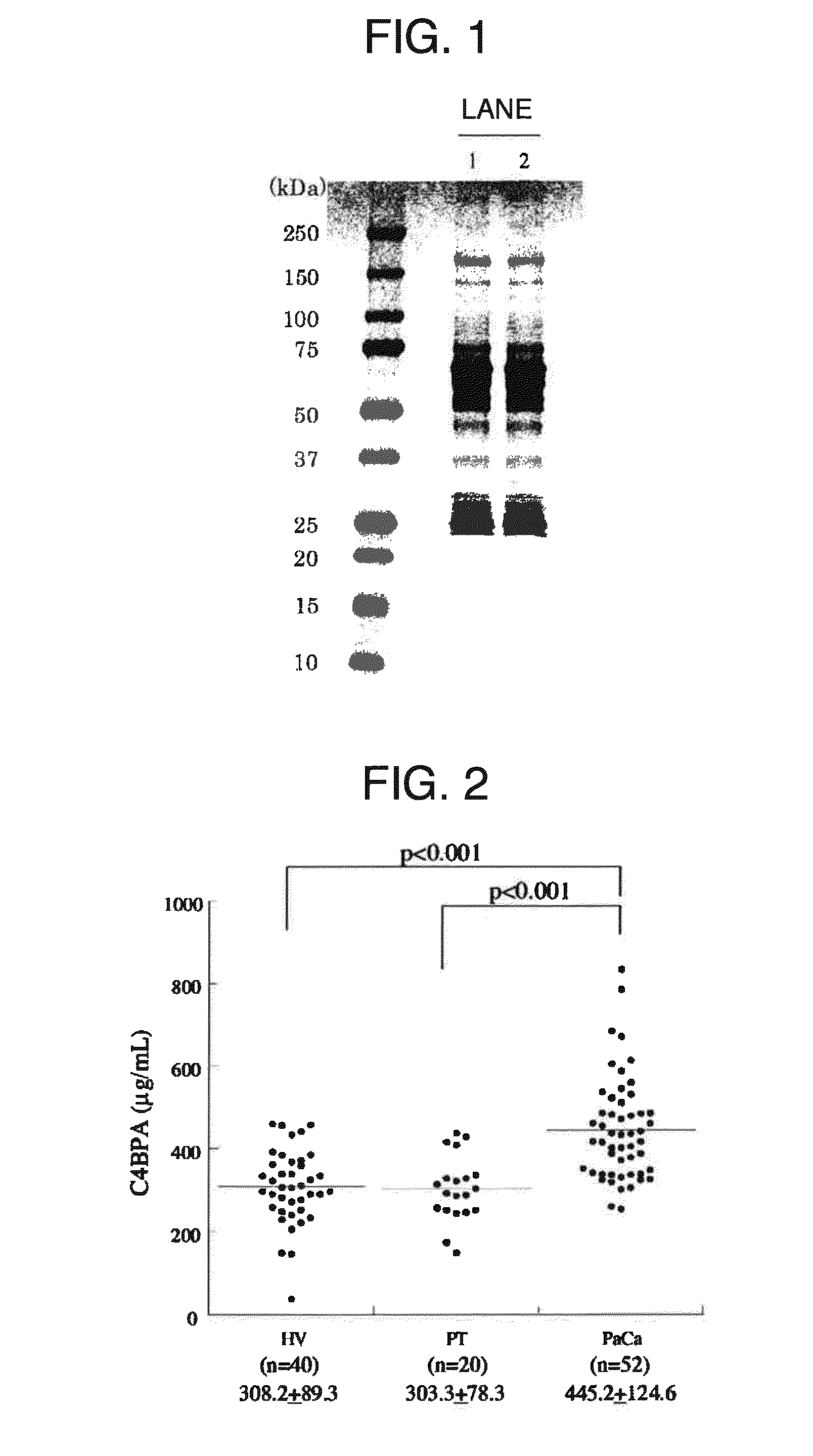
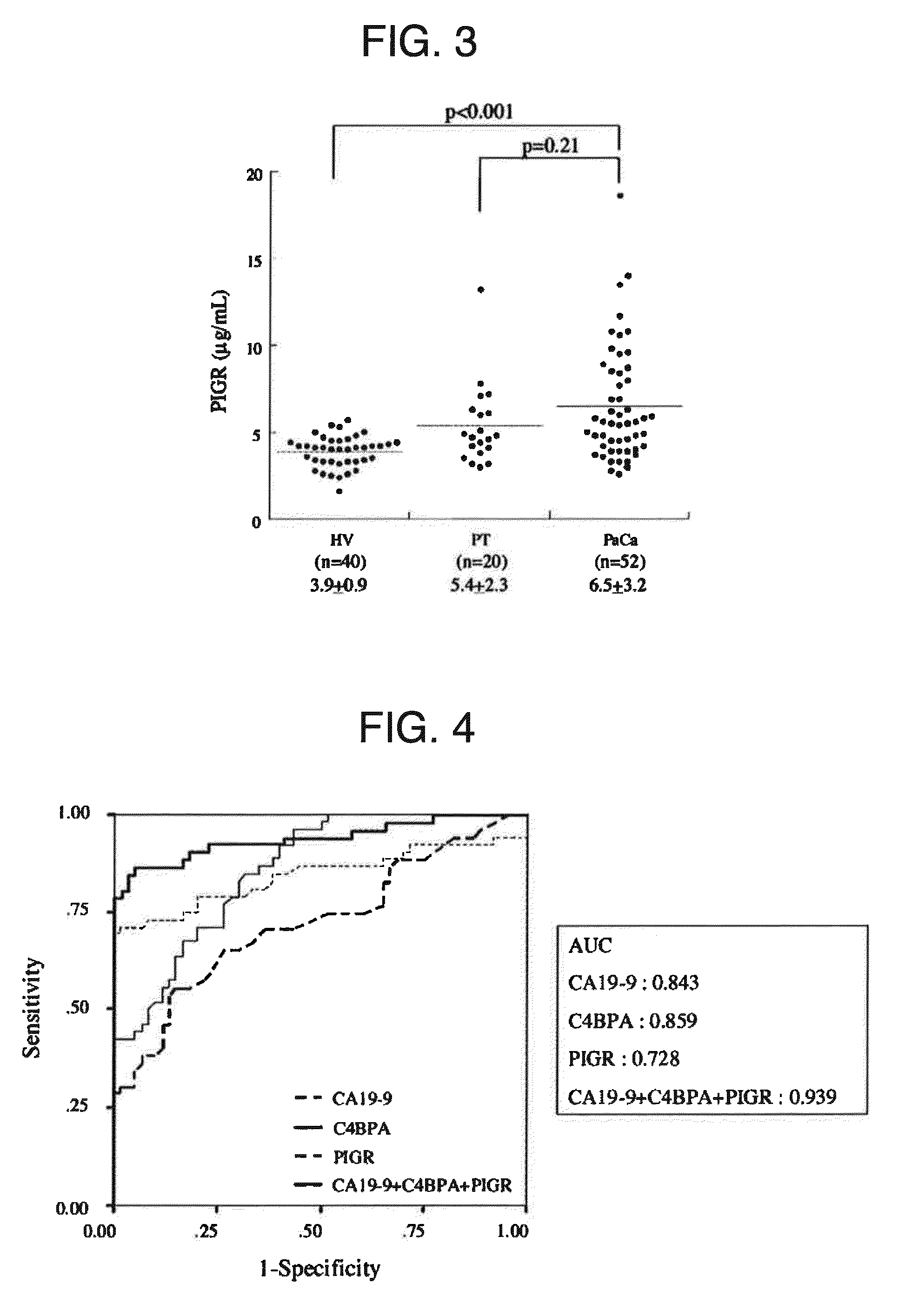
A marker for detecting pancreatic cancer, said marker comprising a glycoprotein C4BPA or PIGR, whereby a pancreatic cancer patient can be distinguished from a chronic pancreatitis patient or a normal subject and thus specifically diagnosed. Also, the marker is usable in monitoring the postoperative prognosis of a pancreatic cancer patient. When the aforesaid marker is used in combination with another pancreatic cancer marker such as CA19-9, furthermore, a pancreatic cancer patient can be distinguished from a chronic pancreatitis patient or a normal subject and diagnosed more specifically.
Owner:NITTO BOSEIKI CO LTD
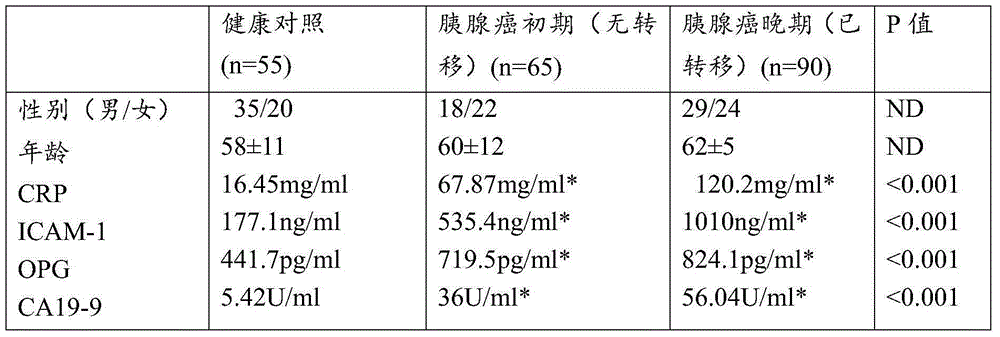
Pancreatic cancer protein biomarker detection kit and detection system
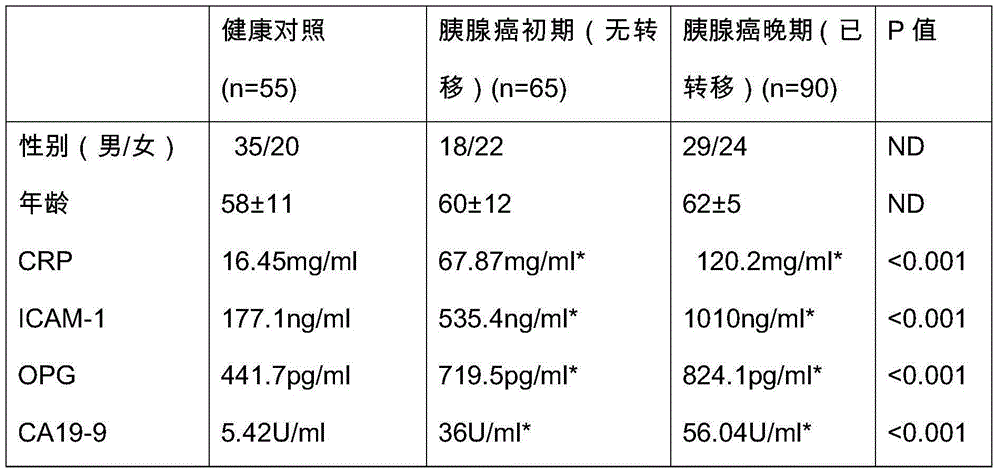
The invention provides a pancreatic cancer protein biomarker detection kit including a CRP monoclonal antibody, an ICAM-1 monoclonal antibody, an OPG monoclonal antibody, a CA19-9 monoclonal antibody, and an enzyme-labelled secondary antibody for forming double antibody sandwich. The invention also provides a pancreatic cancer detection system comprising the detection kit. According to a detection chip and the detection kit, four pancreatic cancer protein biomarkers of CRP, ICAM-1, OPG and CA19-9 obtained through screening by means of 'subtraction method' are subjected to immunization to respectively prepare the specific CRP monoclonal antibody,the specific ICAM-1 monoclonal antibody, the specific OPG monoclonal antibody and the specific CA19-9 monoclonal antibody to be used as the basis, and the enzyme-labelled secondary antibody with double antibody sandwich is combined, and through antigen-antibody specific combination and combination of the enzyme-labelled secondary antibody with double antibody sandwich, biomarking proteins are detected, and higher accuracy and specificity are achieved.
Owner:BIO TECH ACADEMY CHINA +1
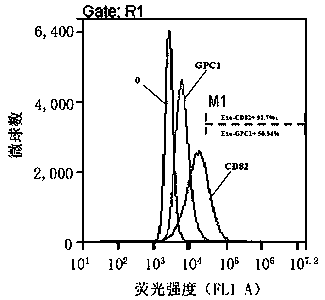
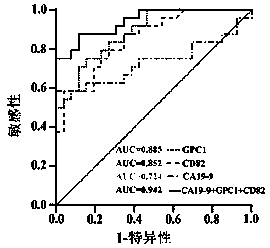
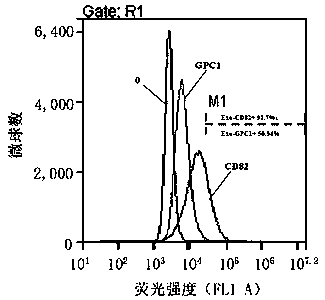
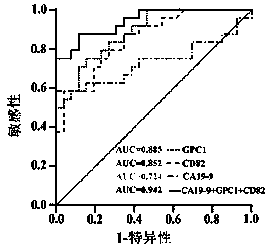
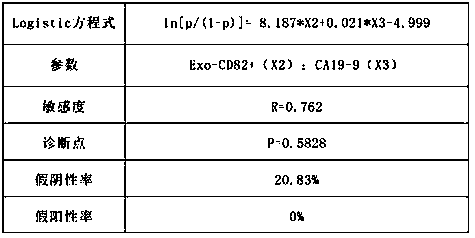
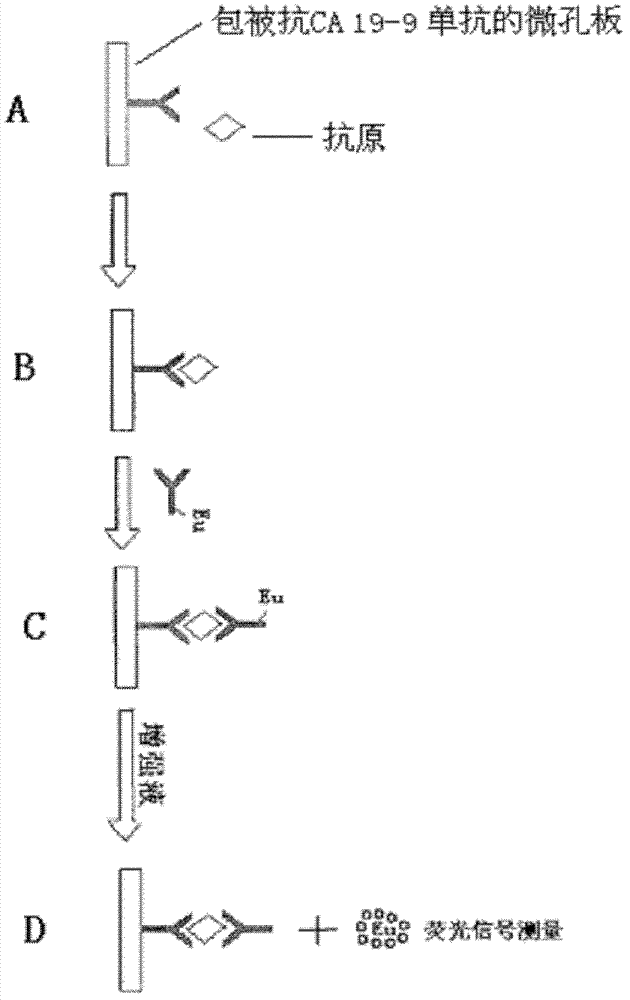
Exosome protein CD82, GPC1-combined CA19-9 for pancreatic cancer early diagnosis and curative effect monitoring
The invention relates to the technical field of medical biological detection, in particular to a multi-index combined detection, a detection kit and a detection method for early diagnosis and curativeeffect monitoring of pancreatic cancer. The indexes comprise an exosome CD82 protein expression quantity, an exosome GPC1 protein expression quantity and CA19-9 content. The latest exosome protein markers are combined for detection, so that the defects of relatively low detection sensitivity and specificity of the single index CA19-9 are overcome, so that the diagnosis result is more accurate andeffective. The method provides a novel method for early diagnosis and curative effect monitoring detection of pancreatic cancer, and is used for realizing early diagnosis and early treatment of pancreatic cancer, the diagnosis and treatment efficiency is improved, the life of a patient is prolonged, and a foundation is laid for improving the prognosis.
Owner:SHANGHAI BIOTECAN PHARMA +1
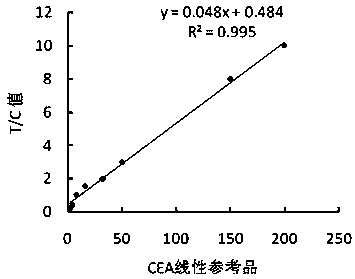
Methods for purifying selected CEA family member proteins
InactiveUS6949629B2Immunoglobulin superfamilyPeptide preparation methodsAntibody AffinitiesCross reacting antigen
Improved methods are provided for purifying selected carcinoembryonic antigen (CEA) family member proteins. Disclosed method steps include cation-exchange chromatography below pH 4.0 and size-exclusion chromatography, and do not include use of perchloric acid or antibody affinity steps. The resulting purified proteins are of at least 90% purity, substantially free of cross-reacting antigens, substantially free of CA19-9, substantially free of endotoxins, and substantially free of antibodies. Purities of greater than 98% have been achieved. Purified CEA family member proteins used as reference standards, in pharmaceutical carriers, and formulated as vaccines are disclosed. The purification, compositions, and use of CEA family member proteins containing altered immunogenic epitopes are also disclosed.
Owner:ASPENBIO PHARMA
Gene chip for detecting pancreatic cancer and application thereof
InactiveCN101993949AMake up detection sensitivityCompensation characteristicsNucleotide librariesMicrobiological testing/measurementNucleotideMortality rate
The invention discloses a gene chip for detecting pancreatic cancer, comprising a solid phase vector and a probe, wherein the probe is hybridized with nucleotide sequences of related pancreatic cancer genes to be detected and / or complementary sequences of the nucleotide sequences; the related pancreatic cancer genes to be detected comprise P21, smad4, P16, c-Myc, bc1-2, Fas, CA19-9, SERPINB9, RHOT1 and P53 gene. The invention further discloses a method for detecting the pancreatic cancer by applying the gene chip. The gene chip of the invention can be used for performing effective detection at the early period of generation and development of the pancreatic cancer so as to improve cure rate and reduce mortality rate.
Owner:SHANGHAI EAST HOSPITAL
Pancreatic cancer protein biomarker and application and detection chip thereof, and detection device
The invention provides a pancreatic cancer protein biomarker and an application thereof, wherein the marker comprises CRP, ICAM-1, OPG and CA19-9 which stably exist in human serum / plasma and can be detected. The pancreatic cancer protein biomarker is obtained through screening in a way of low abundance difference research, and validation indicates that the biomarker participates in generation and development processes of pancreatic cancer, is a believable pancreatic cancer early biomarker, and has higher specificity and sensitivity compared with conventional detection markers. on the basis, the invention further provides a detection chip aiming at the pancreatic cancer protein biomarker and a detection device including the same; according to the detection chip, a specific CRP monoclonal antibody, a specific ICAM-1 monoclonal antibody, a specific OPG monoclonal antibody and a specific CA19-9 respectively prepared through immunization of the CRP, ICAM-1, OPG and CA19-9 are used as the basis and are fixed on a solid-phase matrix to form the chip; through antigen-antibody specific combination, biomarking proteins are detected, and higher accuracy and specificity are achieved.
Owner:BIO TECH ACADEMY CHINA +1
Application of serum protein marker combination to colorectal cancer screening, diagnosis and treatment
The invention belongs to the technical field of molecular biology and clinical detection, and relates to a protein combination, wherein the protein combination is selected from any two or three or four or five or six of CA19-9, CEA, HGF, IP-10, MIP-1beta and SDF-1alpha. The invention further relates to a ligand combination specifically bound with proteins in the protein combination, and application of the protein combination or the ligand combination to preparation of a kit for colorectal cancer screening, auxiliary diagnosis, treatment monitoring and prognosis judging. A serum protein markercombination can be used for colorectal cancer screening, auxiliary diagnosis, treatment monitoring and prognosis judging and particularly used for early colorectal cancer screening and auxiliary diagnosis, and the sensitivity of the serum protein marker combination is superior to that of a single protein marker and commonly used clinical serum markers CA19-9 and CEA in detecting.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Exosomal protein CD82, gpc1 combined with ca19-9 for early diagnosis and curative effect monitoring of pancreatic cancer
The invention relates to the technical field of medical biological detection, in particular to a multi-index combined detection, a detection kit and a detection method for early diagnosis and curativeeffect monitoring of pancreatic cancer. The indexes comprise an exosome CD82 protein expression quantity, an exosome GPC1 protein expression quantity and CA19-9 content. The latest exosome protein markers are combined for detection, so that the defects of relatively low detection sensitivity and specificity of the single index CA19-9 are overcome, so that the diagnosis result is more accurate andeffective. The method provides a novel method for early diagnosis and curative effect monitoring detection of pancreatic cancer, and is used for realizing early diagnosis and early treatment of pancreatic cancer, the diagnosis and treatment efficiency is improved, the life of a patient is prolonged, and a foundation is laid for improving the prognosis.
Owner:SHANGHAI BIOTECAN PHARMA +1
Carbohydrate antigen 19-9 time-resolved immunofluorescence assay and kit
ActiveCN103308683BImprove stabilityHigh analytical sensitivityMaterial analysisAntigenCarbohydrate antigen
The invention discloses a time resolution immunofluorescence analyzing method and a kit for saccharides antigen 19-9. The analyzing method comprises the following steps of: 1, preparing a solid-phase antibody; 2, preparing a lanthanide ion labeled antibody; and 3, adding a standard product or a sample to be measured of the saccharides antigen 19-9 into a solid-phase material with a solid-phase antibody prepared in the step 1, performing reaction on a buffer liquid, and after the solid-phase material is incubated and washed, adding the diluted labeled antibody and incubating again to perform fluorescence detection. The invention also provides the corresponding kit. The method and the kit have the characteristics of high sensitivity, high specificity and high stability, short detection time and high coincidence rate of detecting national quality control products of CA19-9.
Owner:瑞孚迪生物医学(上海)有限公司
Liquid phase chip for joint detection of multiple tumor markers and preparation method thereof
The invention relates to a liquid phase chip for joint detection of multiple tumor markers and a preparation method thereof. The liquid phase chip comprises micro-balloons of a coupled antibody (at least two of the followings: AFP, CEA, CA125, CA153, CA19-9, CA242, CA72-4, PSA, HGH, Beta-HCG), corresponding biotin-labeled detection antibodies, streptavidin phycoerythrin and vegetable hydrosol or polysaccharide hydrosol which has a solute content of 1-10 wt per thousand and does not contain protein. The preparation of the liquid phase chip comprises refining and purification of hydrosol, coupling between captured antibodies and micro-balloons, preparation of biotin-labeled antibodies, dispersing the coupled micro-balloons into the vegetable hydrosol or the polysaccharide hydrosol, and the like. The liquid phase chip has the advantages of stable performance, good micro-balloon dispersivity, long preservation time, fast and convenient use and operation, small amount of samples in use, high detection sensitivity, wide linear scope and low detection cost, can detect ten tumor markers at most at one time, and requires a cost which is a quarter of the total fee of conventional methods.
Owner:HENAN YUKANG BIOTECH
CA15-3, CEA, CA19-9, CA12-5, SF mammary cancer colloidal gold five joint inspection diagnostic reagent kit
The invention discloses a diagnosis kit for breast cancer and preparation method thereof. Five kinds of tumor markers for diagnosis of breast cancer such as CA15-3, CEA, CA19-9, CA12-5, SF are detected together at the same test paper and using immunity chromatography technique the monoclonal antibodies for CA15-3, CEA, CA19-9, CA12-5, SF are fixed on the cellulose nitrate film, at the same time the five kinds of monoclonal antibodies are marked by gold particles of different size and the said gold antibodies are mixed to produce gold affixture pad, finally the gold affixture pad is assembled with a sample treating pad to form a test paper. The said kit quickly test the five kinds of antigens in human whole blood or serum, plasm using double antibody sandwiching method, so as to conquer many defects of previously separation detecting method, only one drop of blood can detect five tumor markers and the detection result is more direct and the sensitivity and specificity of detection is greatly increased.
Owner:BEIJING MOKOBIO LIFE SCI CO LTD
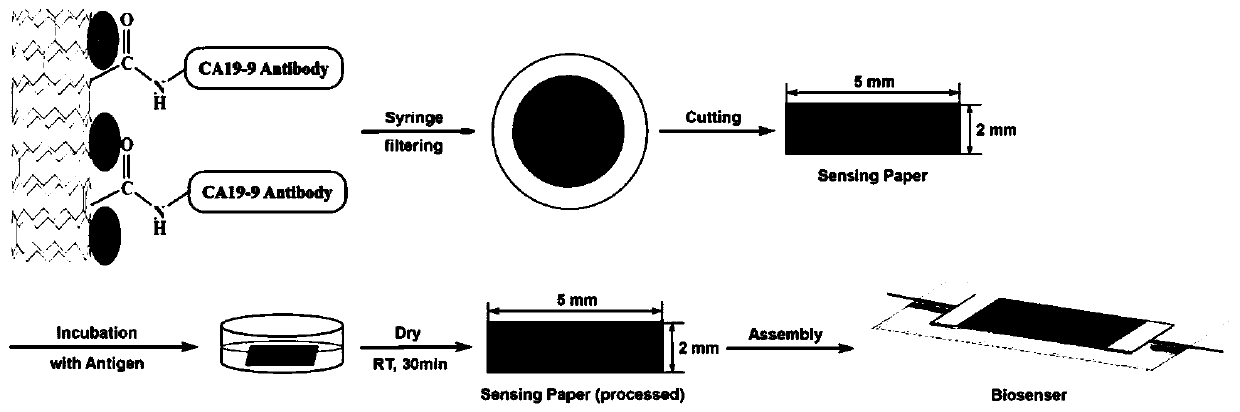
Paper-based sensor element for detecting cancer antigens and preparation method of paper-based sensor element
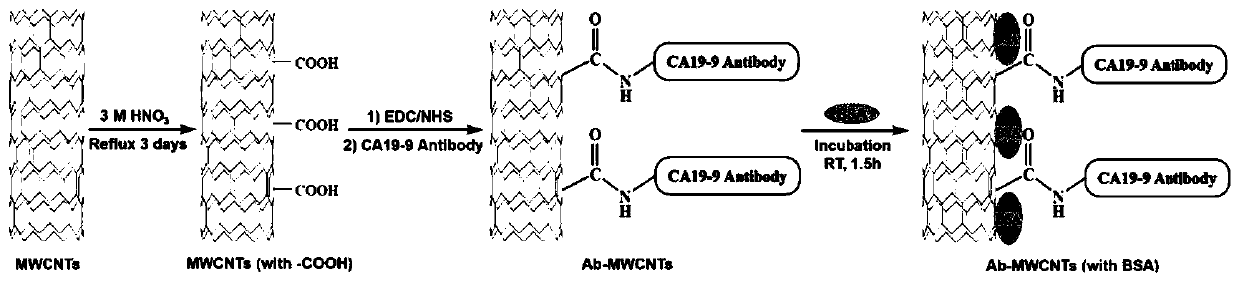
The invention discloses a paper-based sensor element for detecting cancer antigens and a preparation method of the paper-based sensor element. The paper-based sensor element comprises a filter paper base a carboxylated carbon nanotubes; the plurality of the carboxylated carbon nanotubes are deposited on the filter paper base; and the carboxylated carbon nanotubes are specifically bound with a specific CA19-9 antibody of pancreatic cancer cells. The paper-based sensor element of the invention can convert antigen concentration information into directly measurable electrical signals, has the advantages of low manufacturing cost, high precision and high stability, and can realize economical and rapid cancer antigen screening; the sensor of the invention has good detection performance under a low CA19-9 concentration range (from 0 to 40U / ml), can detect pancreatic cancer in an early stage; and the detection time of the sensor of the invention only takes about two hours, and the detection efficiency of the sensor is about 12 times higher than that of the ELISA (enzyme-linked immune sorbent assay).
Owner:XI AN JIAOTONG UNIV
Gene chip for detecting pancreatic cancer and application thereof
InactiveCN101993949BConducive to screeningHigh cure rateNucleotide librariesMicrobiological testing/measurementNucleotideMortality rate
Owner:SHANGHAI EAST HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com