Time resolution fluorescence immunochromatography test strip and kit for jointly detecting CA19-9 and Carcino-Embryonic Antigen (CEA)
A technology of time-resolved fluorescence and immunochromatography test strips, which is applied in the direction of analytical materials, biological testing, measuring devices, etc., can solve problems such as inappropriate diagnosis, and achieve the effects of stable quality, simple equipment, and good detection performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Carboxyl group fluorescent microspheres are used as composite raw materials, polystyrene-carboxyeuropium chelate, the solid content is 1%, the particle size is 210nm, the maximum emission wavelength is 613nm, and the maximum excitation wavelength is 365nm.
[0034] Wash the time-resolved fluorescent microspheres twice with activation buffer (0.05M boric acid buffer at pH 8.0), resuspend with activation buffer, add carbodiimide, shake and activate at 30°C for 60min, add 20ul of 10% ethanol After mixing, centrifuge and wash to remove carbodiimide, add labeling buffer (0.05M boric acid buffer at pH 8.0), mix well, then add 80ug CA19-9 monoclonal antibody and 65ug CEA monoclonal antibody, shake at 37°C Mark for 2 hours, and after centrifugation, use reconstitution buffer (0.01MTris-Hcl (pH8.5), 0.1%BSA, 0.1%T-20, 0.1%PVP, 0.1%PEG20000, 3% trehalose, 0.05%NaN 3 ) resuspended, added 50ul of 10% BSA to block for 30min, and obtained time-resolved fluorescent microsphere-labeled...
Embodiment 2
[0036] CA19-9 monoclonal antibody (denoted as S1 (subtype IgG2a, titer 1:128), S2 (subtype IgG3, titer 1:256), S3 (subtype IgG2a, titer 1 :256), S4 (subtype IgG1, titer 1:128)) and CEA monoclonal antibody (denoted as M1 (subtype IgG3, titer 1:128), M2 (subtype IgG2a, potency 1:128) 1:256), M3 (subtype IgG2a, titer 1:128), M4 (subtype IgG2a, titer 1:64)) were provided by Henan Bioengineering Technology Research Center. The method in Example 1 was used to couple with time-resolved fluorescent microspheres to prepare labeled CA19-9 monoclonal antibody complexes (referred to as S1 complex, S2 complex, S3 complex, and S4 complex) and CEA monoclonal antibody complexes. Clone antibody complexes (denoted M1 complexes, M2 complexes, M3 complexes, M4 complexes). The labeled CA19-9 monoclonal antibody complex and CEA monoclonal antibody complex were mixed and sprayed on glass fiber cotton, and the CA19-9 monoclonal antibody and CEA monoclonal antibody were respectively coated on the nit...
Embodiment 3
[0042] Example 3: Preparation of time-resolved fluorescent immunochromatographic test strips for joint detection of CA19-9 and CEA
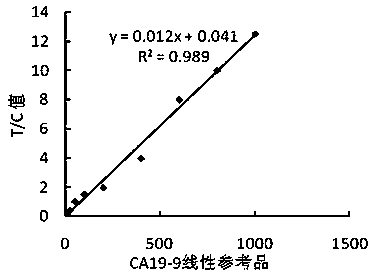
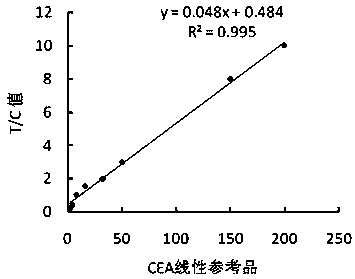
[0043] (1) Using the method in Example 1, prepare time-resolved fluorescent microsphere-labeled CA19-9 monoclonal antibody S2 complex and CEA monoclonal antibody M1 complex;
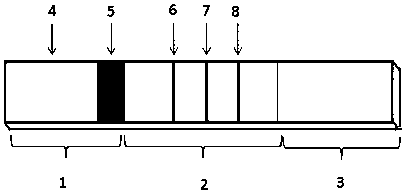
[0044] (2) Antibody coating of detection line and quality control line: Paste the nitrocellulose membrane on the middle of the bottom plate, and overlap with the absorbent paper on the upper end by 1-2mm, and use 0.05M PBS, 1%BSA, 0.05% NaN 3 The CA19-9 monoclonal antibody S4, CEA monoclonal antibody M2 and goat anti-mouse polyclonal antibody diluted in the diluent were coated on the T1 line, T2 line and C line position on the nitrocellulose membrane respectively. Under 40% humidity, dry for 4 hours;
[0045] (3) Spraying in the bonding area: Soak the glass fiber cotton in the treatment solution (0.01MTris-Hcl (pH8.5), 0.1%BSA, 0.1%T-20, 0.1%PVP, 0.1%PEG20000, 3%Trehalose...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com