Application of serum protein marker combination to colorectal cancer screening, diagnosis and treatment
A colorectal cancer and protein technology, which is applied in the application field of serum protein marker combination in colorectal cancer screening, diagnosis and treatment, can solve the problems of unsuitability for tumor patients, low sensitivity, and difficult tumor carcinogenesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Example 1: Sensitivity and specificity of protein combinations in serum samples from patients with colorectal cancer
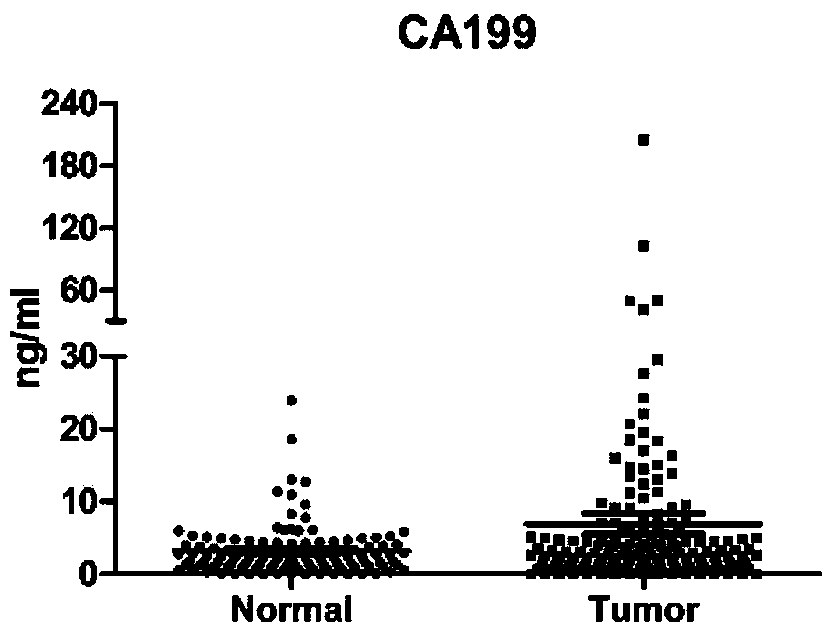
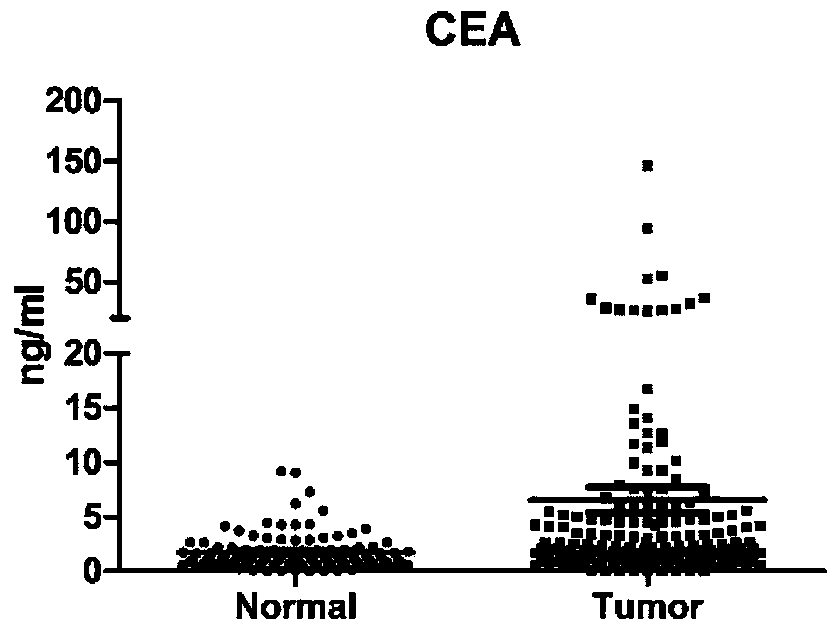
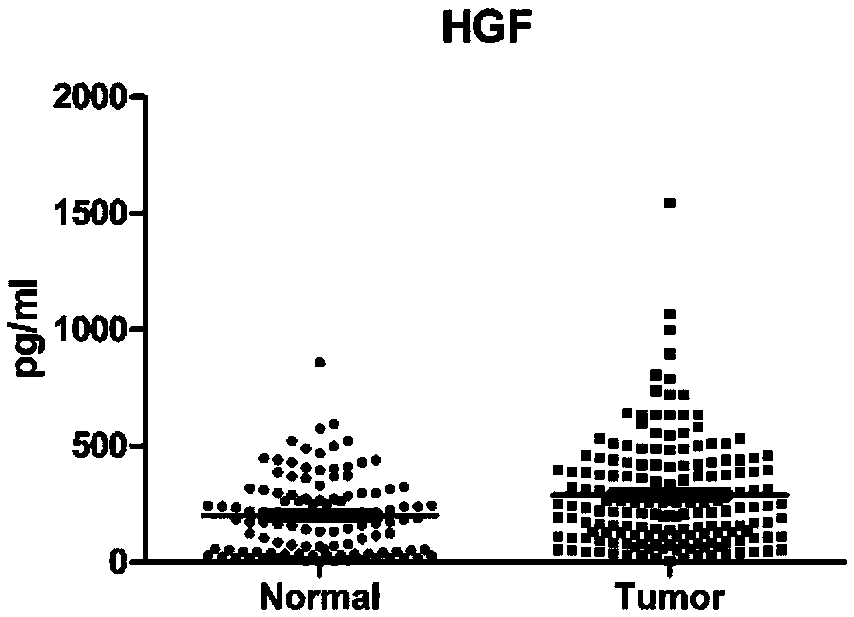
[0079] For 173 patients with colorectal cancer confirmed by histopathological diagnosis (colon cancer patients or rectal cancer patients, not particularly distinguishing early or late stage) and 115 normal human serum samples, ELISA method was used to detect CA19-9, CEA, HGF , IP-10, MIP-1β and SDF-1α six protein levels, and analyzed.
[0080] The experimental results are shown in Table 1, Table 2 and Figure 1A - Figure 1F shown.
[0081] Table 1: Sensitivity and specificity of individual proteins in colorectal cancer
[0082]
[0083]
[0084] All samples are valid samples.
[0085] Table 2: Sensitivity and specificity of protein combinations in colorectal cancer
[0086]
[0087] Note: 6 out of 2 positive means 2 out of 6 antibodies are positive; 5 out of 2 positive means 2 out of 5 antibodies are positive; 2 out of 2 positive means ...
Embodiment 2
[0089] Example 2: Sensitivity of protein-antibody combination in the detection of colon cancer serum samples
[0090] For 24 patients with colon cancer confirmed by histopathological diagnosis, the levels of six proteins, CA19-9, CEA, HGF, IP-10, MIP-1β and SDF-1α, were detected and analyzed by ELISA.
[0091] The experimental results are shown in Table 3 and Table 4 and Figure 2A-Figure 2F shown.
[0092] Table 3: Sensitivity of individual proteins in colon cancer
[0093] protein name
Number of positive cases
sensitivity
CA19-9
8
33.33%
CEA
11
45.83%
HGF
7
29.17%
IP-10
8
33.33%
MIP-1β
6
25.00%
SDF-1α
6
25.00%
[0094] Note: All samples are valid samples.
[0095] Table 4: Sensitivity of protein combinations in colon cancer
[0096]
[0097] Note: 6 out of 2 positive means 2 out of 6 antibodies are positive; 5 out of 2 positive means 2 out of 5 antibodies are positive...
Embodiment 3
[0099] Example 3: Sensitivity of protein-antibody combination in the detection of rectal cancer serum samples
[0100] For 89 patients with rectal cancer confirmed by histopathological diagnosis, the levels of six proteins, CA19-9, CEA, HGF, IP-10, MIP-1β and SDF-1α, were detected and analyzed by ELISA.
[0101] The experimental results are shown in Table 5 and Table 6 and Figure 3A-Figure 3F shown.
[0102] Table 5: Sensitivity of individual proteins in rectal cancer
[0103] protein name
Number of positive cases
sensitivity
CA19-9
30
33.71%
CEA
55
61.80%
HGF
33
37.08%
IP-10
31
34.83%
MIP-1β
33
37.08%
SDF-1α
32
35.96%
[0104] All samples are valid samples.
[0105] Table 6: Sensitivity of protein combinations in rectal cancer
[0106]
[0107] Note: 6 out of 2 positive means 2 out of 6 antibodies are positive; 5 out of 2 positive means 2 out of 5 antibodies are posit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com