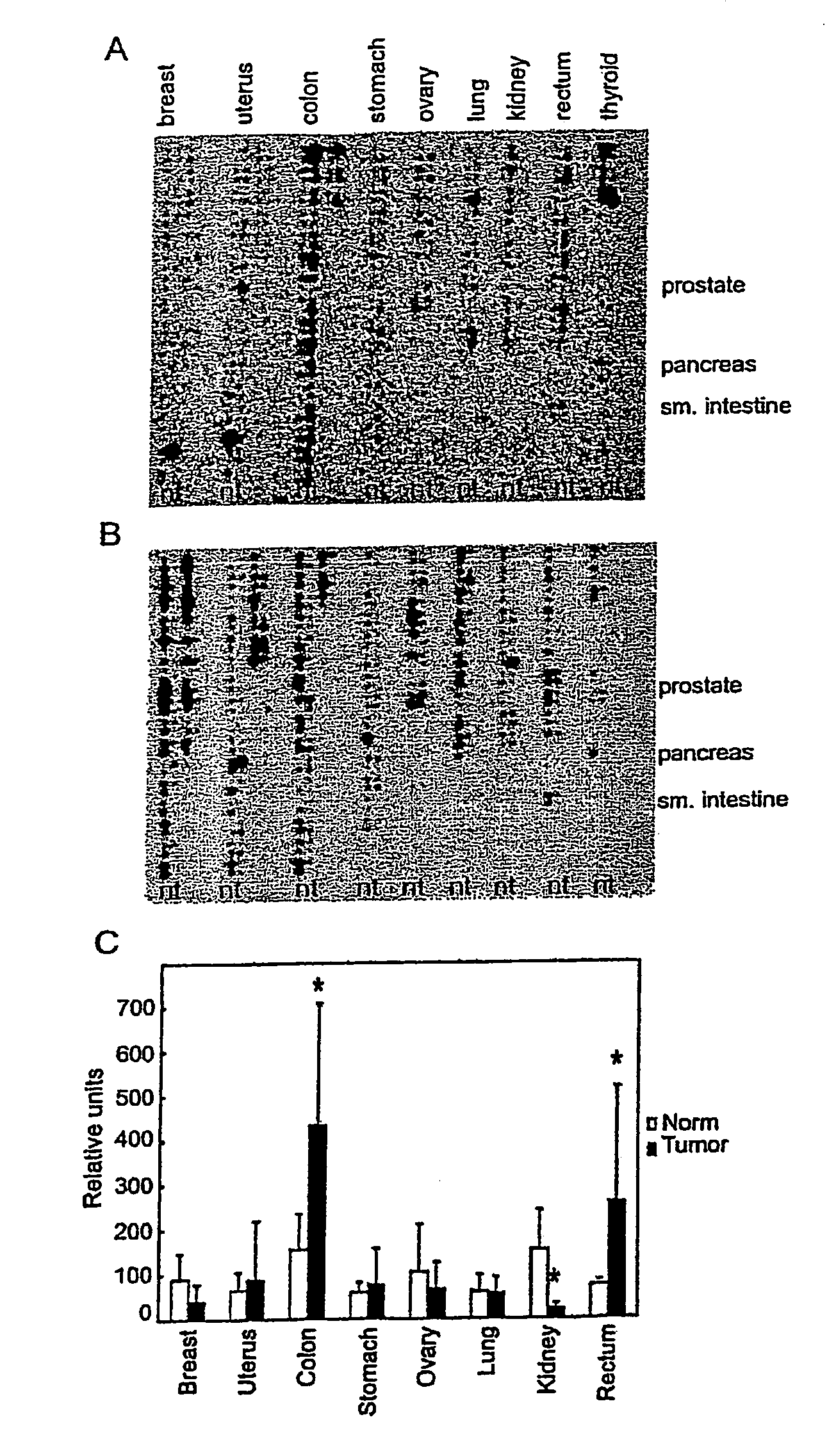
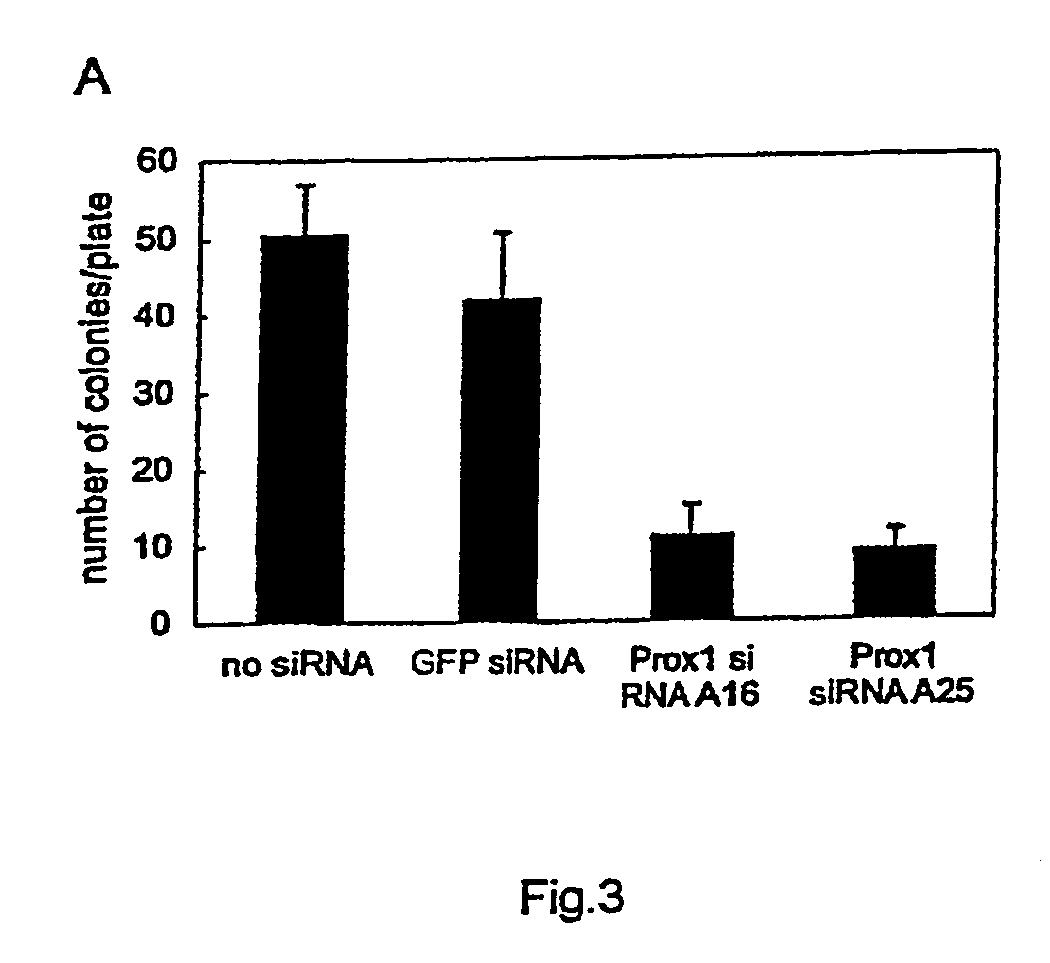
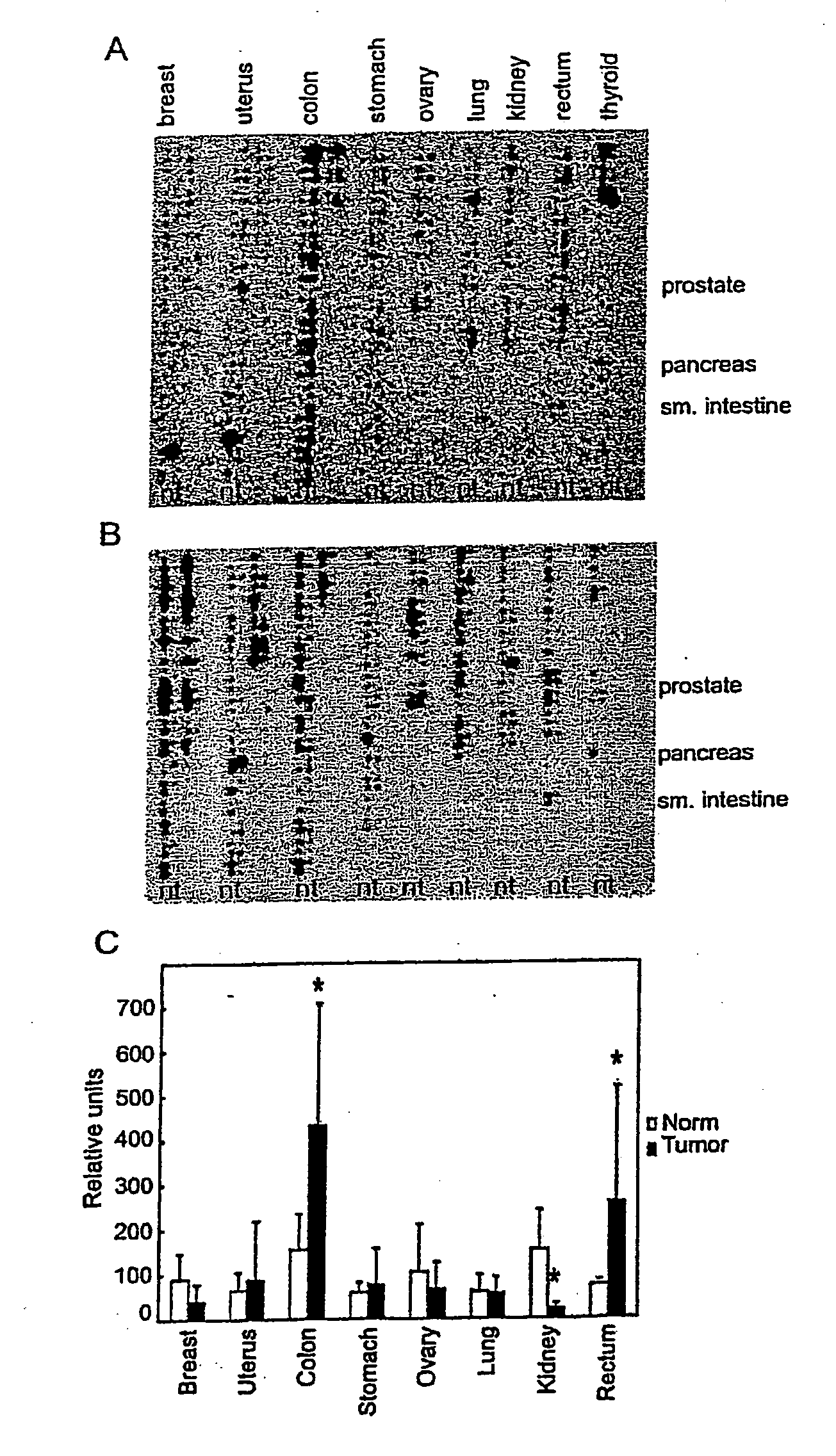
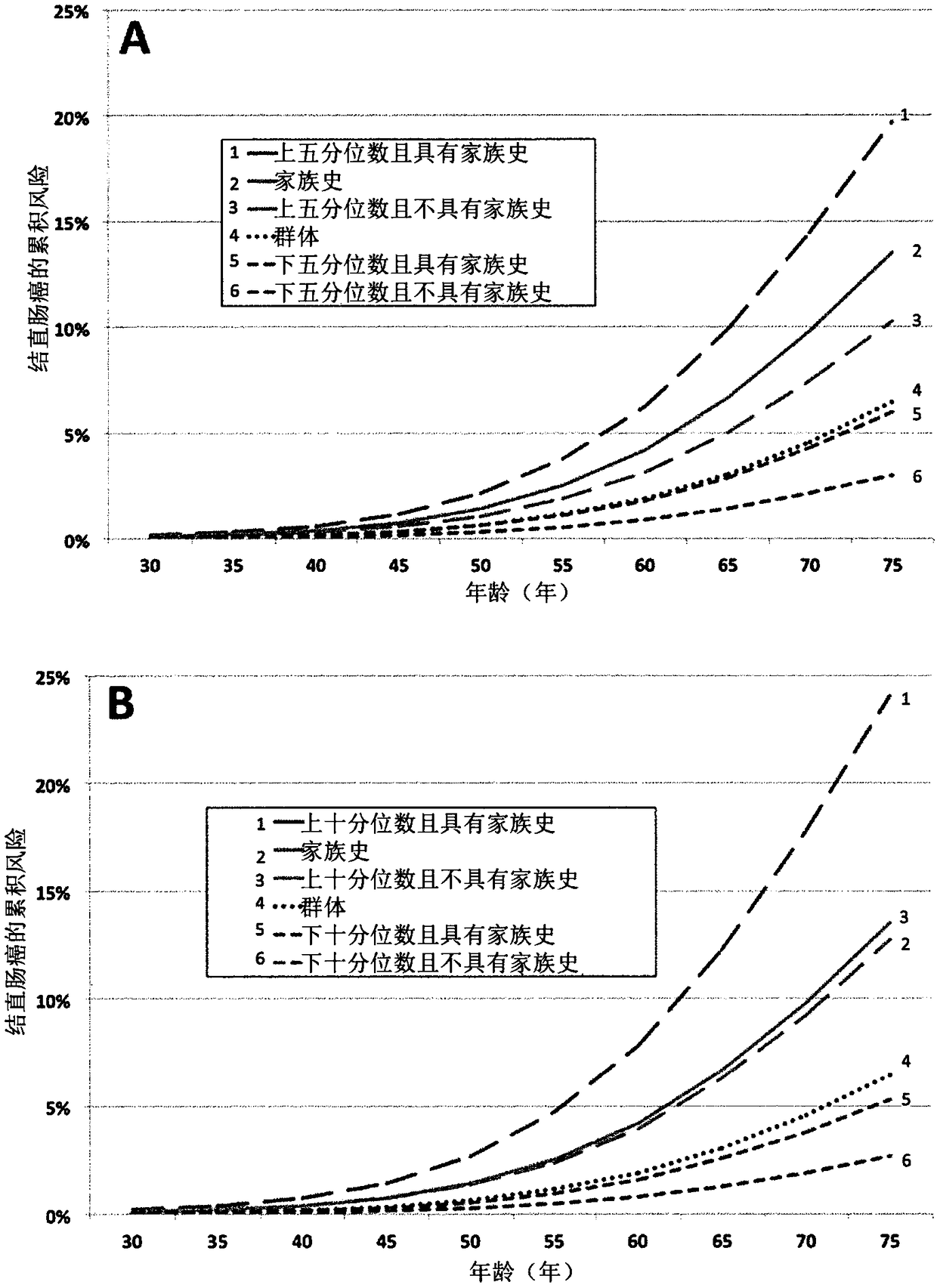
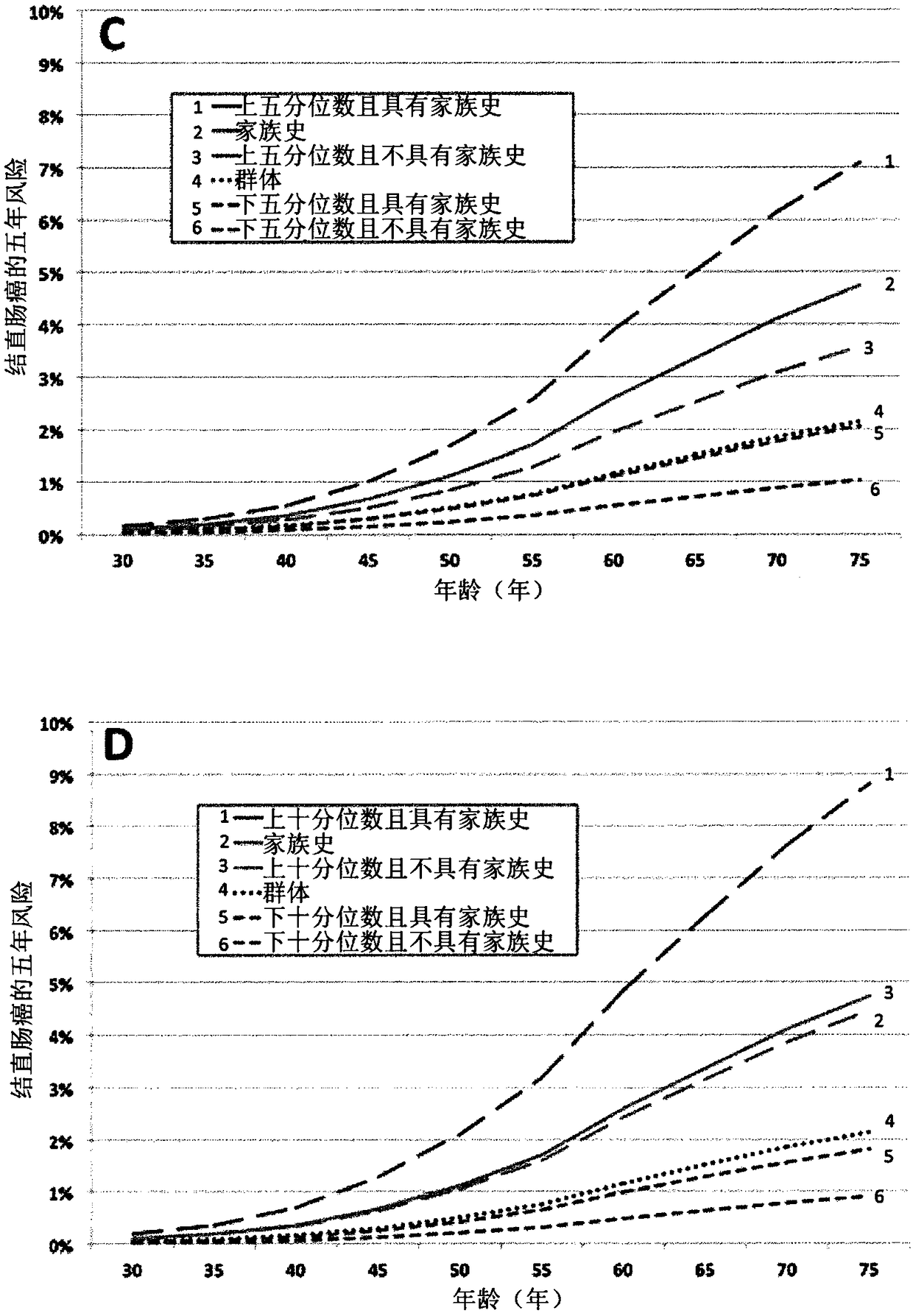
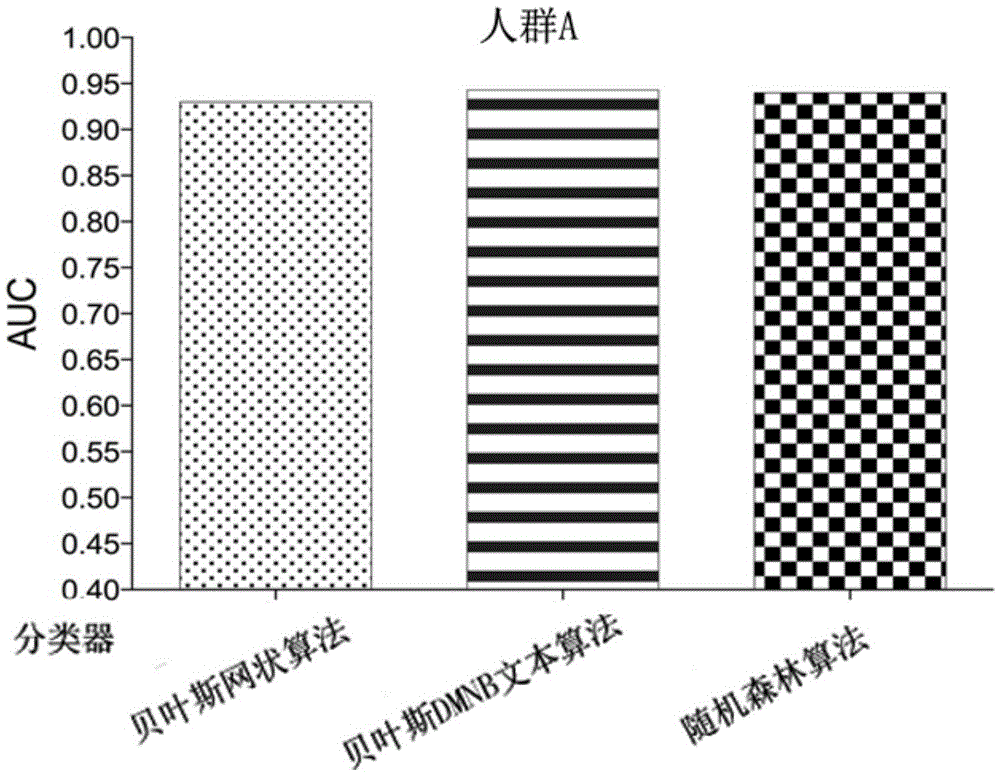
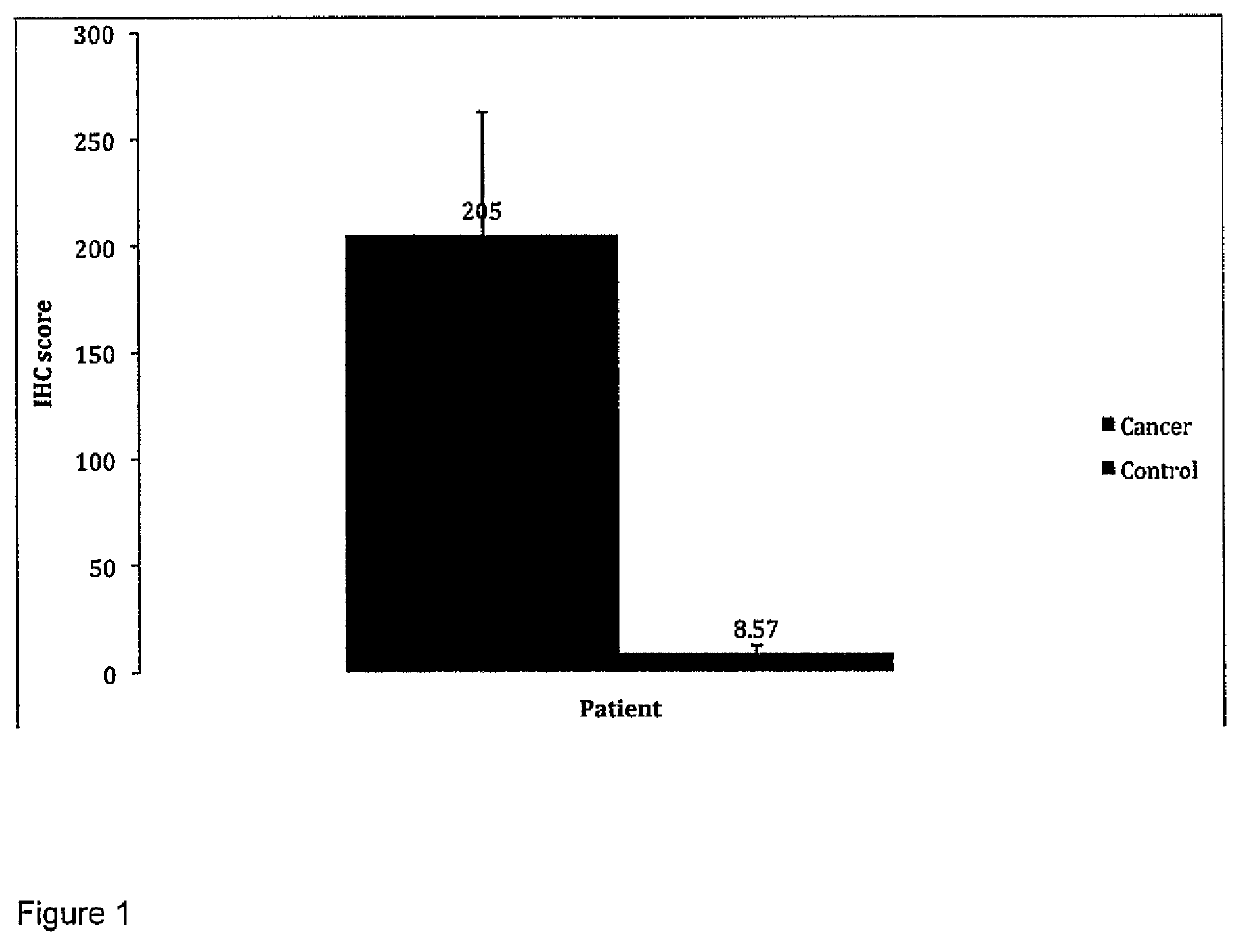
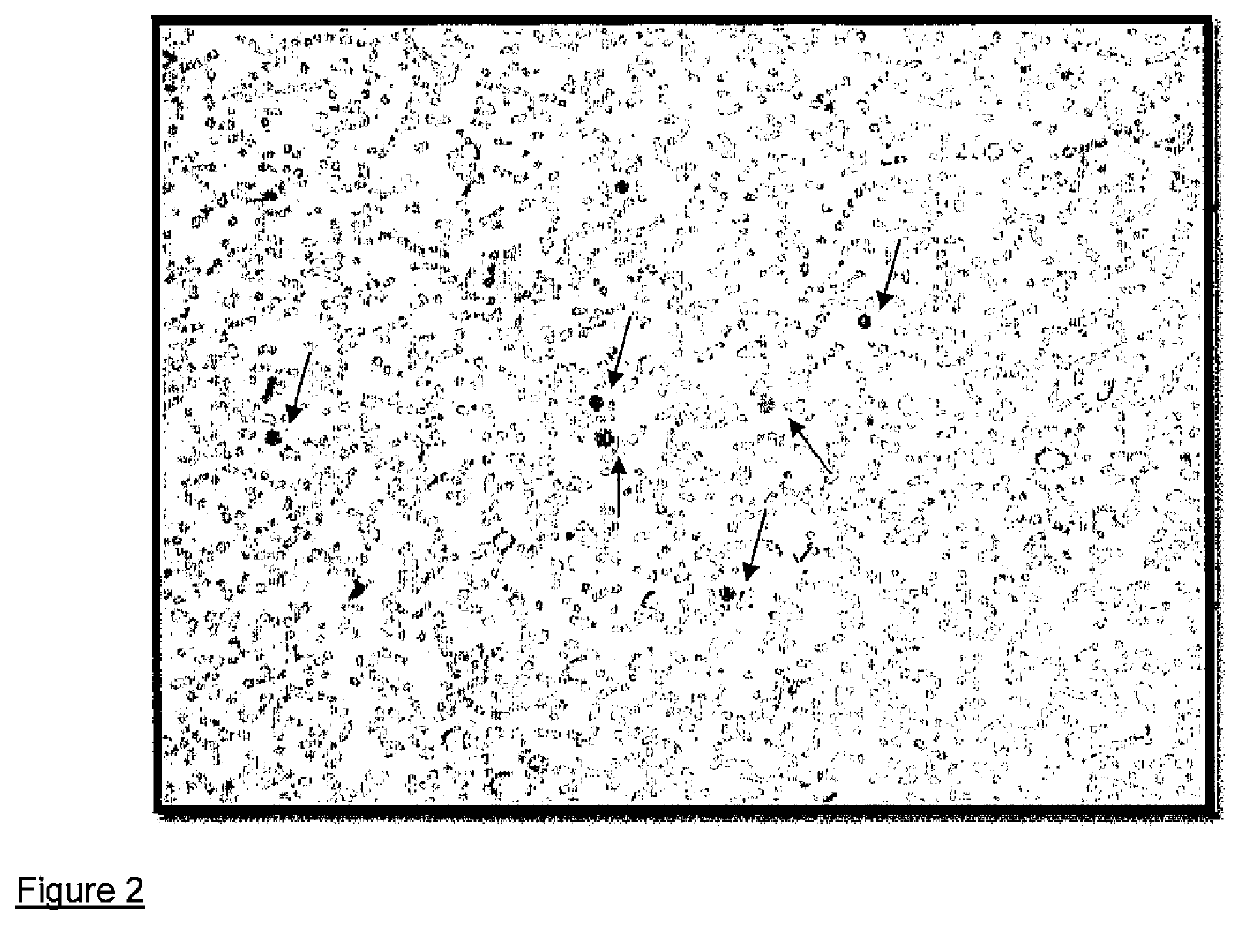
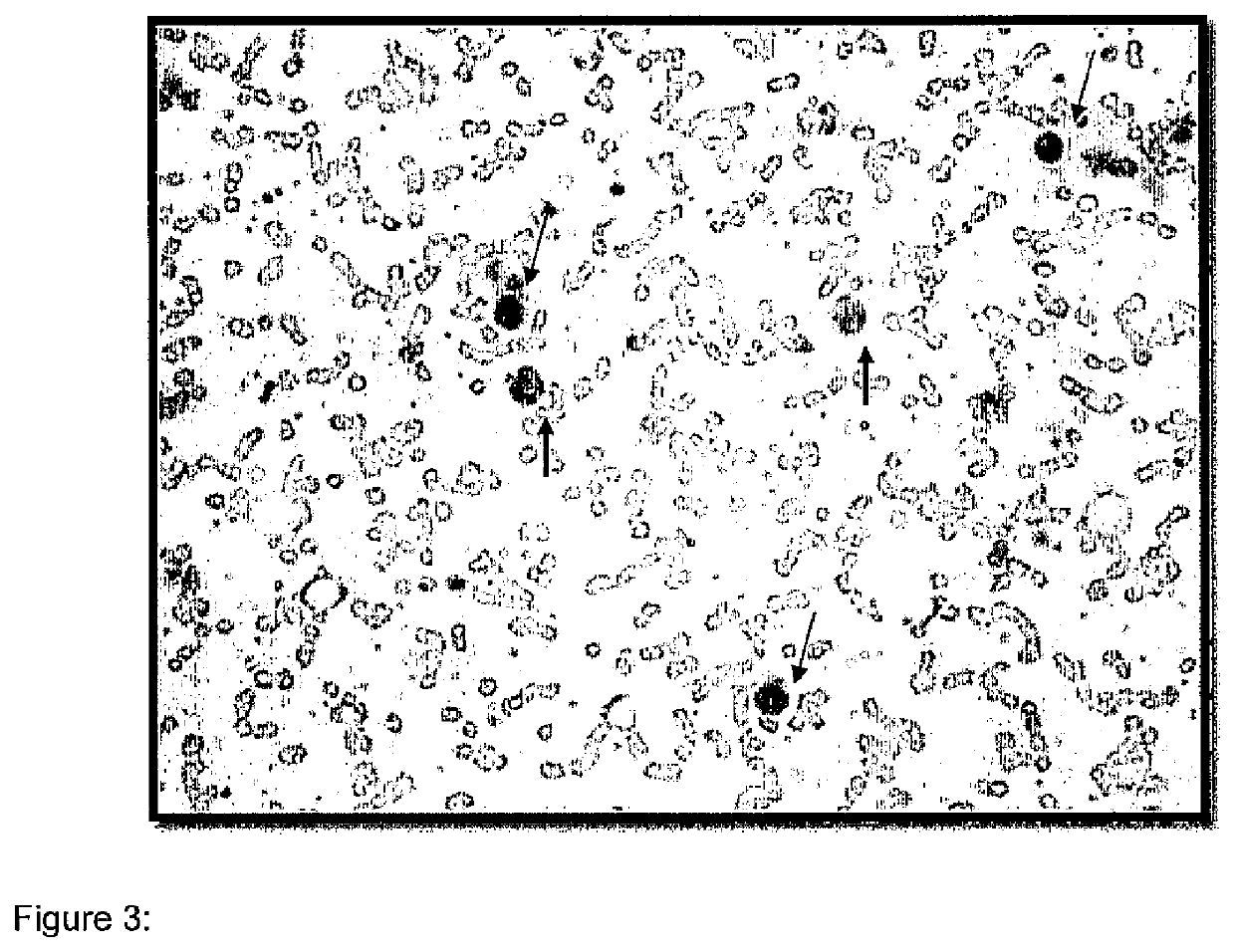
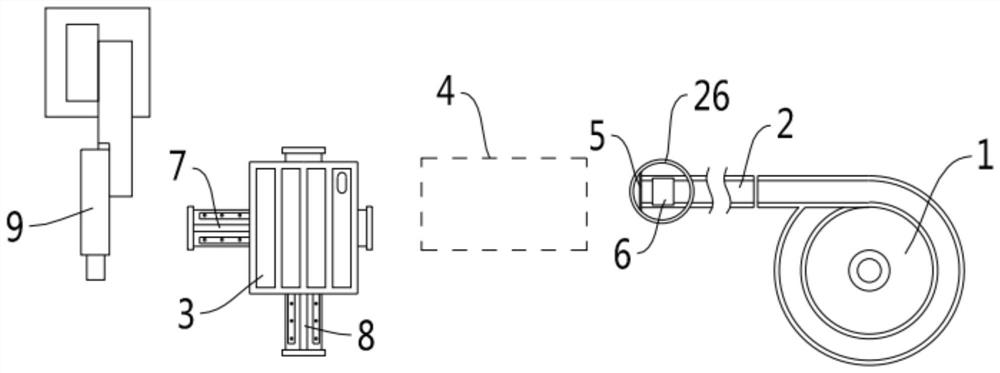
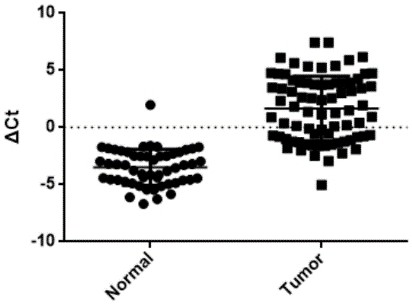
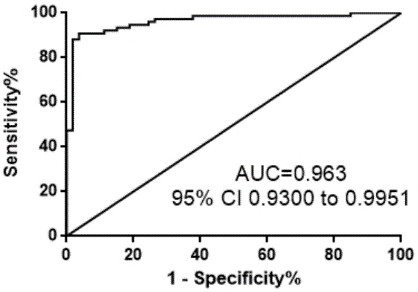
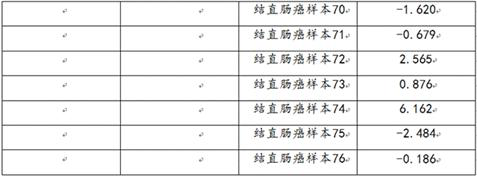
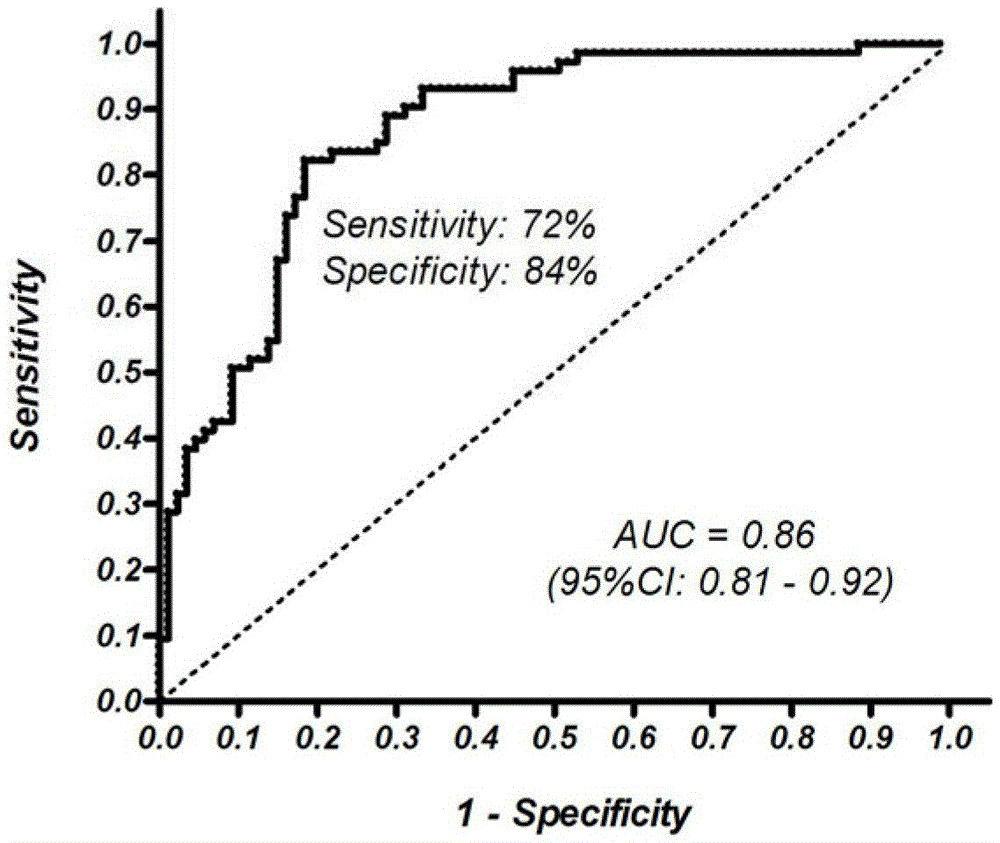
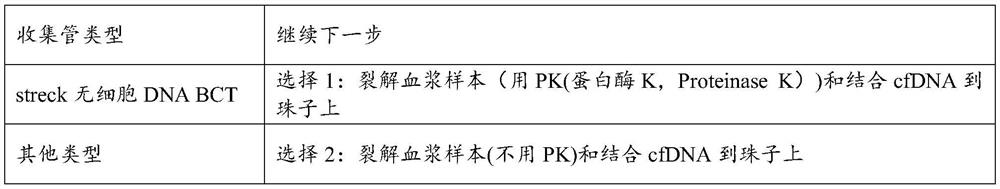
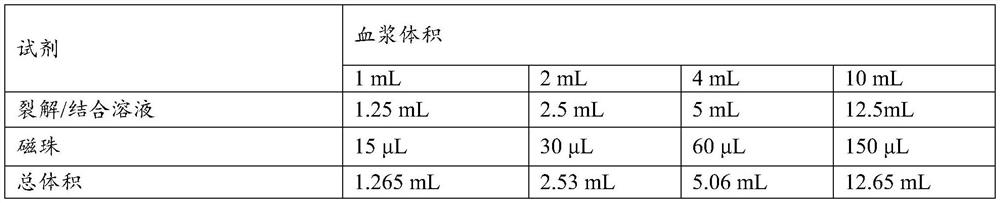
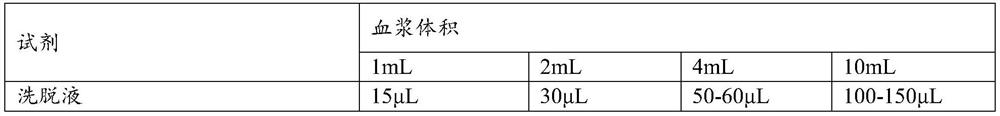
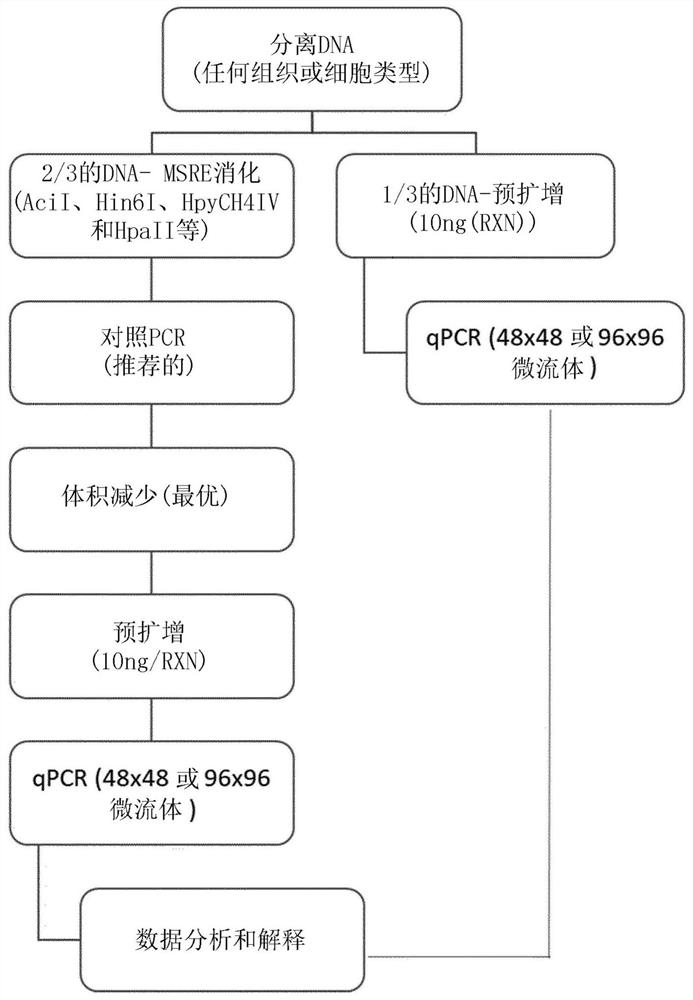
Patents
Literature
34 results about "Colorectal cancer screening" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Materials and methods for colorectal cancer screening, diagnosis and therapy
The invention provides materials and methods for colorectal cancer screening, diagnosis, and therapy.
Owner:LICENTIA LTD
Materials and methods for colorectal cancer screening, diagnosis and therapy
InactiveUS20070026405A1Sugar derivativesMicrobiological testing/measurementProviding materialOncology
The invention provides materials and methods for colorectal cancer screening, diagnosis, and therapy.
Owner:LICENTIA LTD
Method for evaluating stable state of flora in excrement sample and application of method in colorectal cancer screening
PendingCN108690864ALow costGood Gut Health ScreeningMicrobiological testing/measurementMicroorganism based processesClostridium leptumFeces
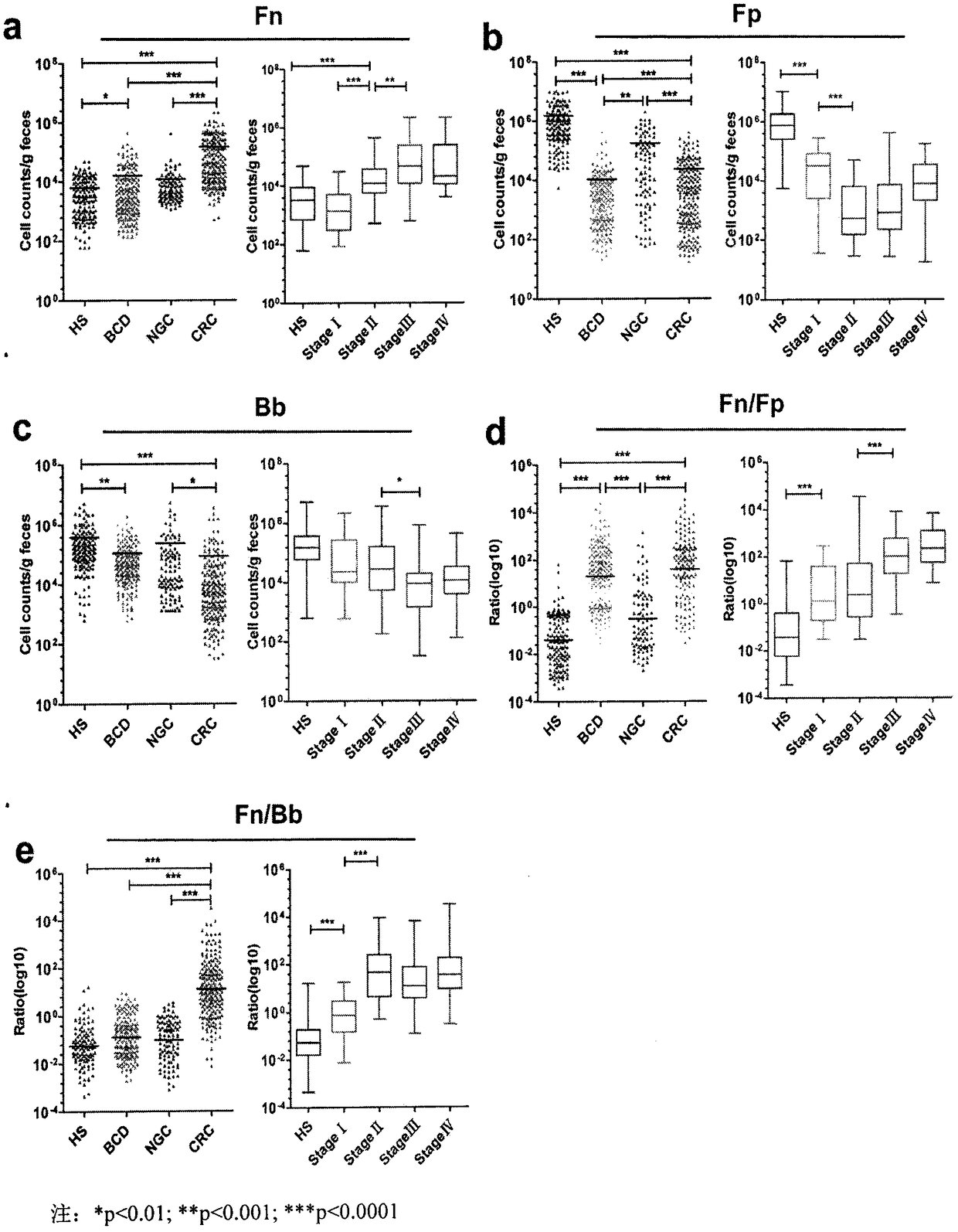
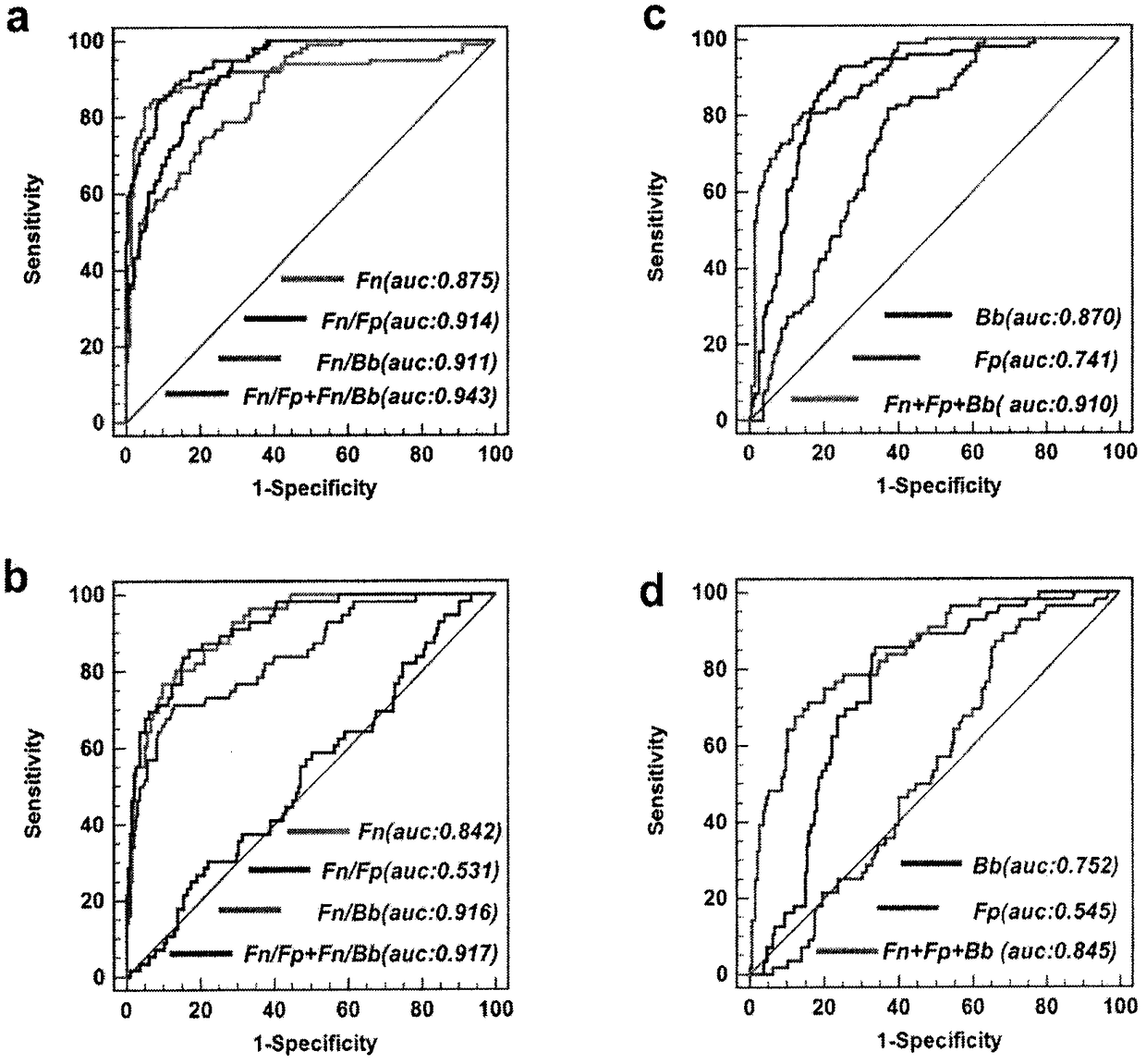
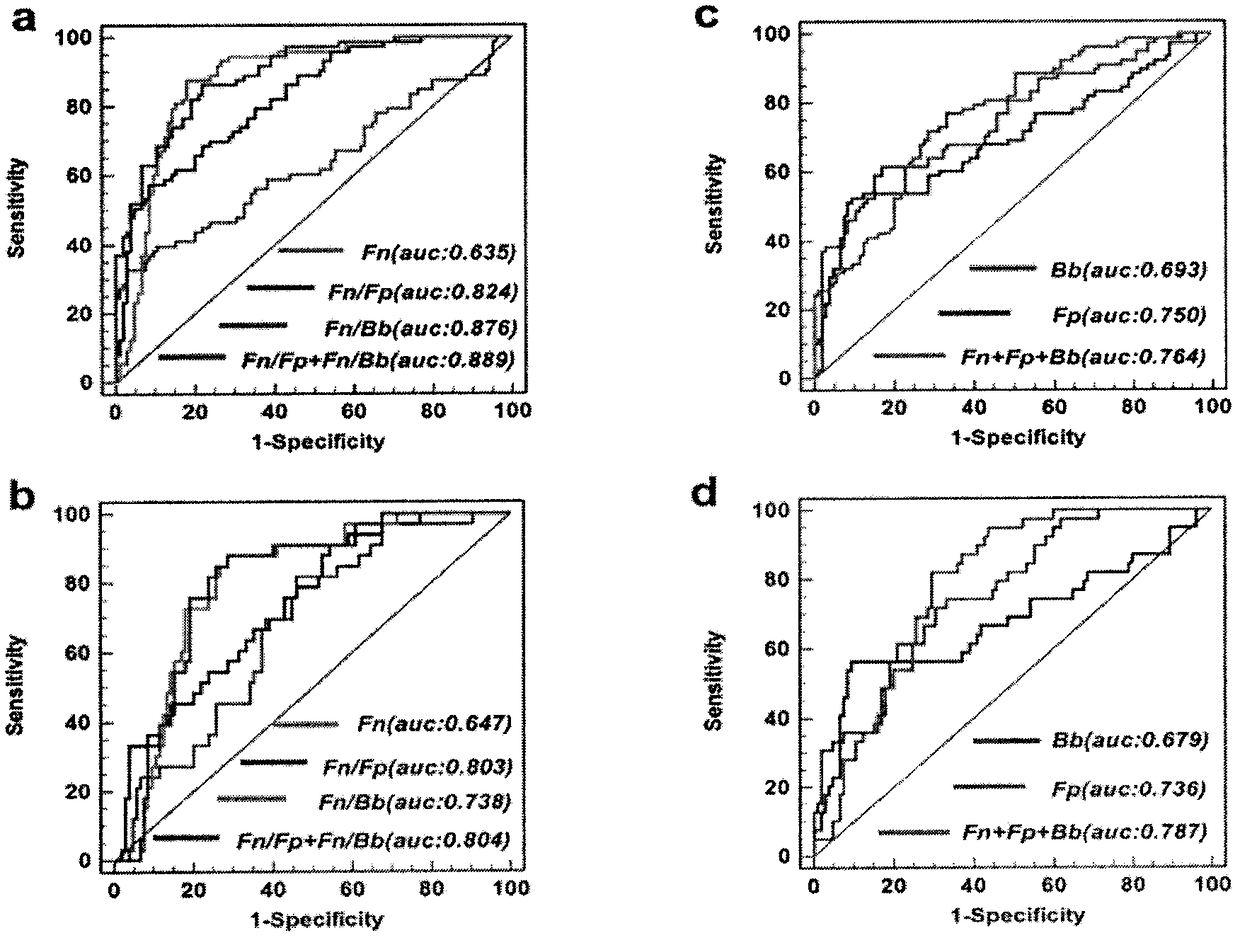
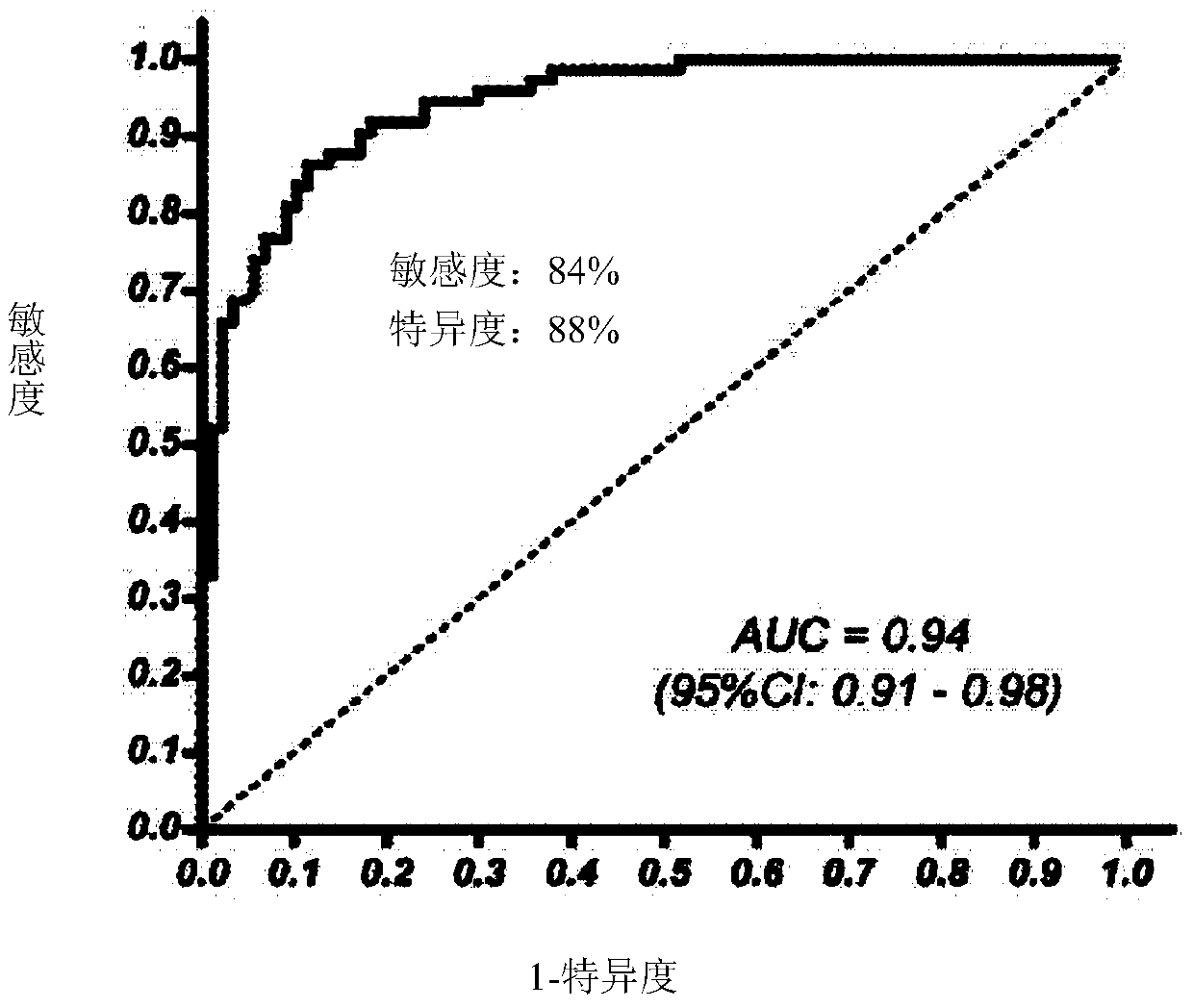
The invention relates to a method for calculating a flora balanced relation index in an individual excrement sample, and an application of the method in screening, diagnosis or auxiliary diagnosis incolorectal cancer (CRC). By extracting bacteria in excrement during DNA sequencing, the types and quantity characteristics of the bacteria can be obtained, and a CRC diagnosis by taking quantity ratiocharacteristic of a plurality of bacteria as a base is carried out. Compared with the methods used in clinical diagnosis or noninvasive screening of CRC with an applied patent, the method is completely noninvasive, and can realize accurate diagnosis of CRC. The analysis result displays that a ratio of fusobacterium nucleatum Fn to bifidobacteria Bb quantity (Fn / Bb) has high susceptibility and specificity on CRC screening, which can respectively reach 84.6% and 92.3% (AUC=0.911). the ratio of fusobacterium nucleatum Fn to clostridium leptum Fp (Fn / Fp) quantity is combined to increase the diagnosis value on CRC, and the Area Under Curve (AUC) of a subject work characteristic curve can reach 0.943. In addition, combination of Fn / Bb and Fn / Fp quantity ratio for screening I-stage CRC has 60% of specificity and 90% of sensitivity.
Owner:SUN YAT SEN UNIV
Reagent for detecting clostridium symbiosum and application thereof
InactiveCN105803061AEasy to judgeIncreased sensitivityMicrobiological testing/measurementMicroorganism based processesBacteroidesFeces
The invention discloses an application of the reagent for detecting clostridium symbiosum in preparing an early colorectal cancer diagnostic kit. According to the diagnostic kit, DNA of bacteria in excrement is extracted and a quantitative polymerase chain reaction is conducted so that the feature of relative abundance of the clostridium symbiosum can be acquired, and diagnosis of early colorectal cancers (limited to the colon cancer and the rectal cancer of submucosa) is conducted with the feature as a basis. Compared with noninvasive colorectal cancer screening methods applied to clinics at present and applied for a patent, the regent is completely noninvasive, operation is relatively easy and low in price, the colorectal cancers can be predicted more accurately, and the area under curve (AUC value) of an ROC curve is 0.70 or above.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
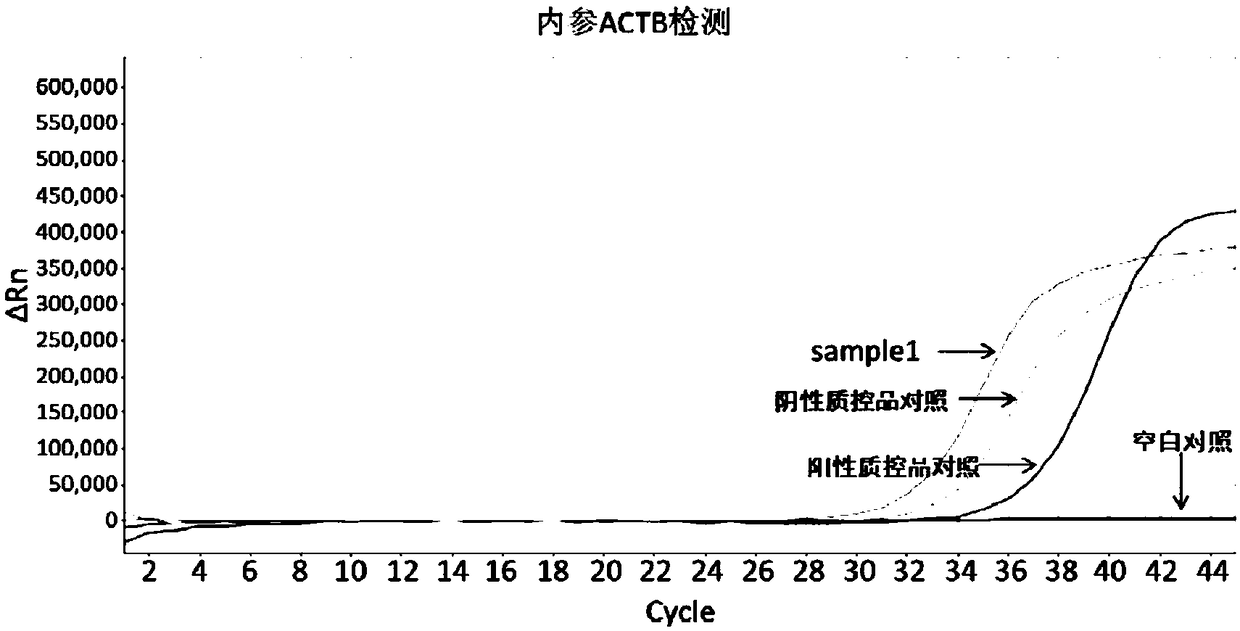
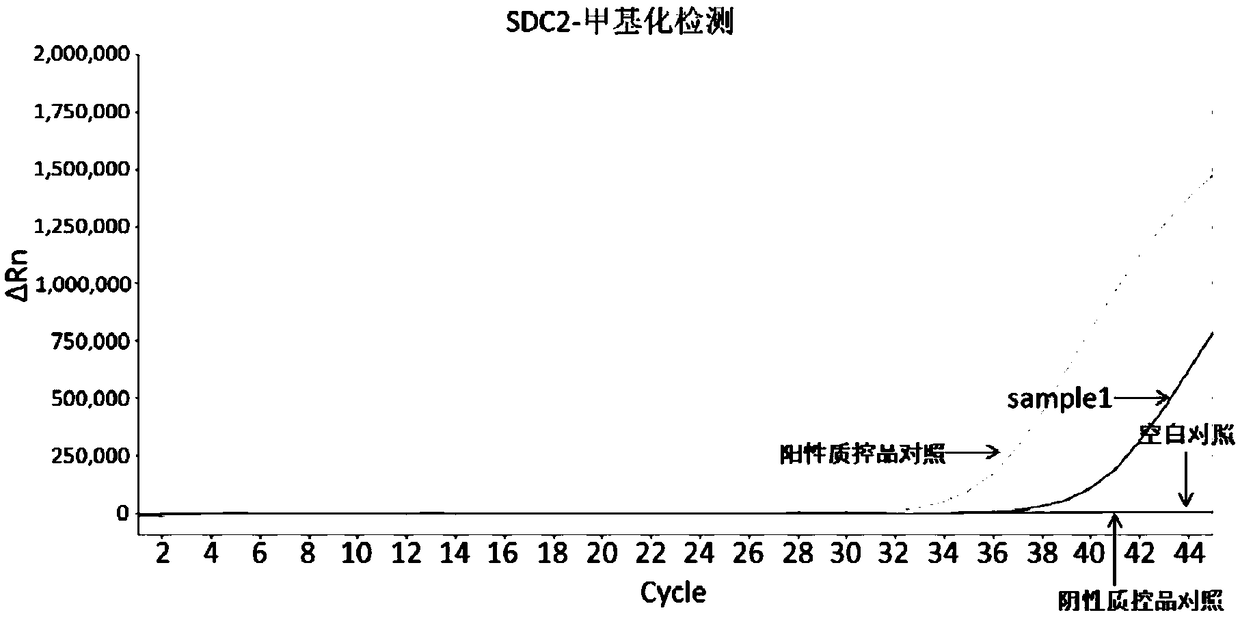
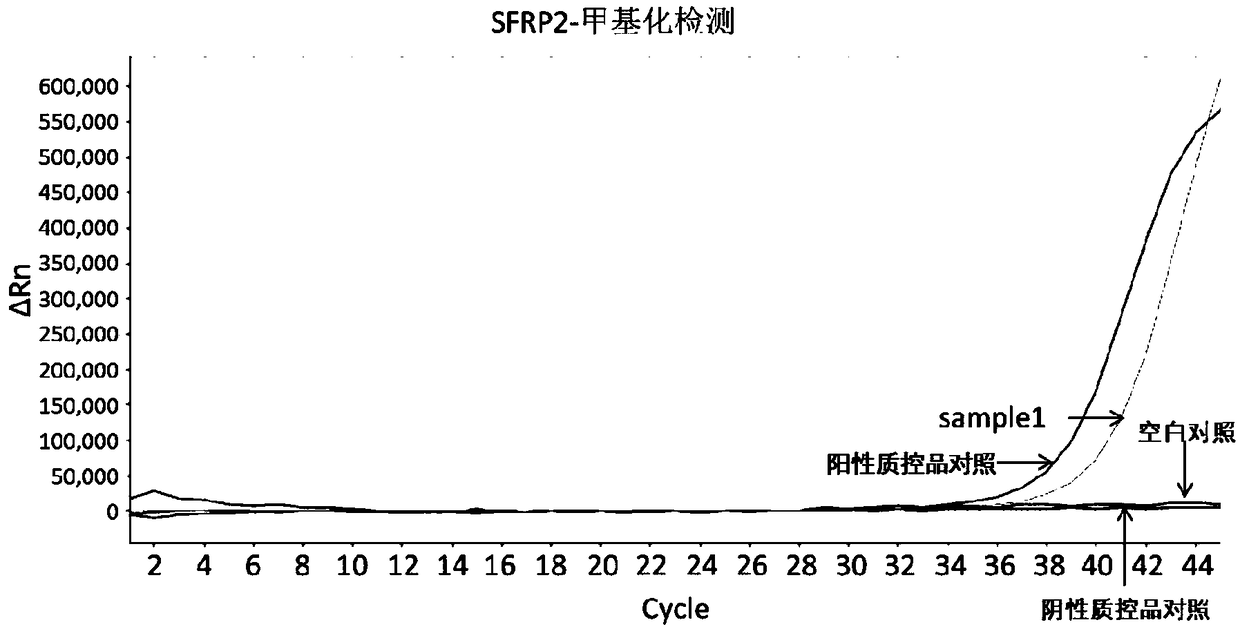
Early diagnosis reagent for colorectal cancer based on combined detection of methylation levels of SDC2 gene and SFRP2 gene
ActiveCN108977543AEasy to getThe sampling process is simple and convenientMicrobiological testing/measurementDiseaseDiagnosis early
The invention belongs to the field of biology, and particularly relates to an early diagnosis reagent for colorectal cancer based on combined detection of methylation levels of a SDC2 gene and a SFRP2gene. The early diagnosis reagent is characterized in that the excrement is used as a detection sample, the SDC2 gene and the SFRP2 gene are jointly used as biological markers, and the methylation levels of the SDC2 gene and the SFRP2 gene are detected. Proofed by results, the early diagnosis reagent has the advantages that the detected methylation levels of the SDC2 gene and the SFRP2 gene in the excrement are highly related with the disease generation of colorectal cancer, the detection sensitivity of colorectal cancer is 94.74%, and the specificity is 96.67%; the detection result is accurate, quick and simple, and the non-wound type colorectal cancer screening and early diagnosis are realized.
Owner:SHANGHAI REALBIO TECH CO LTD
Methods for assessing risk of developing colorectal cancer
PendingCN109072308AFrequent screeningNucleotide librariesMicrobiological testing/measurementOncologyCancer research
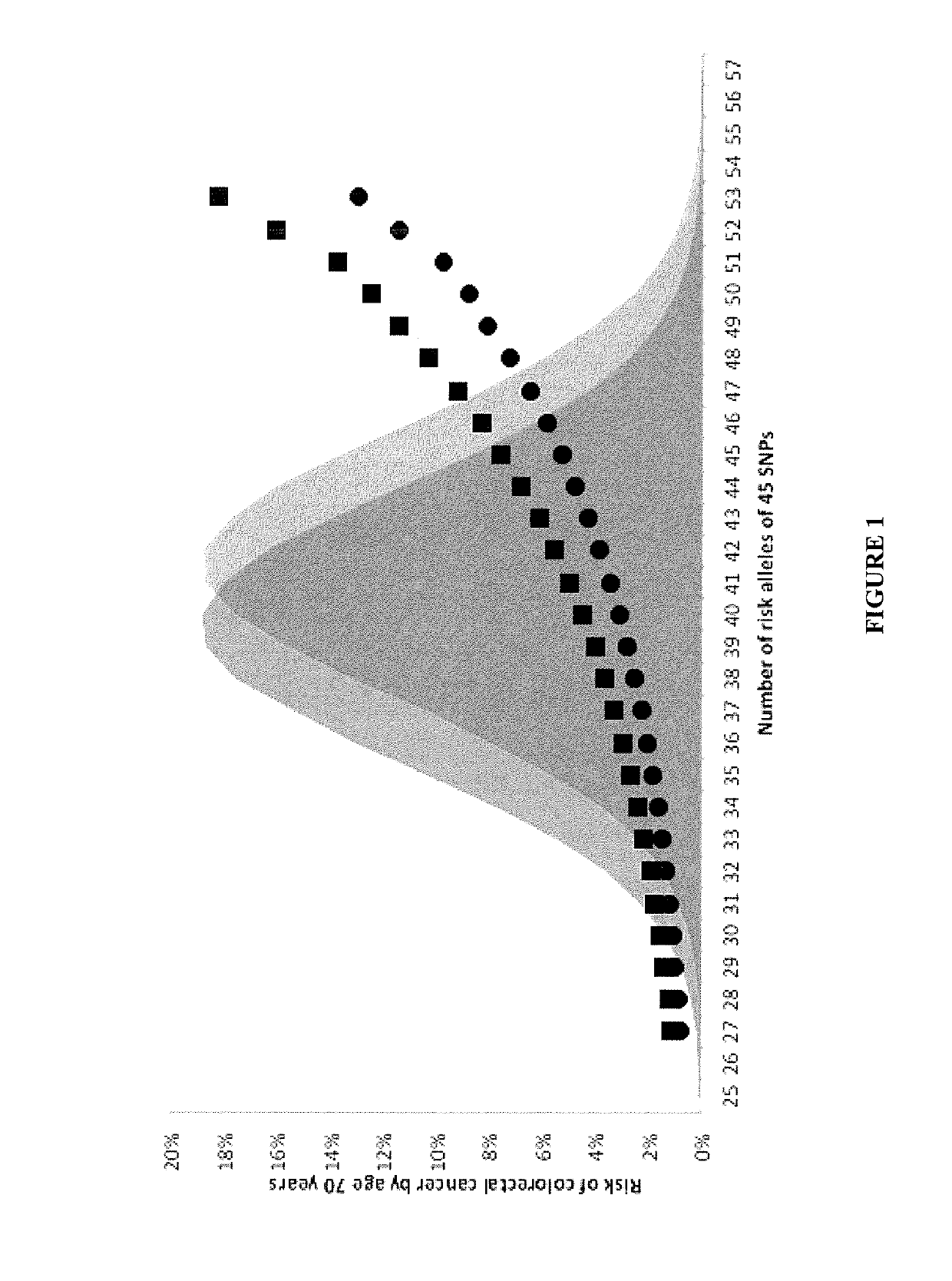
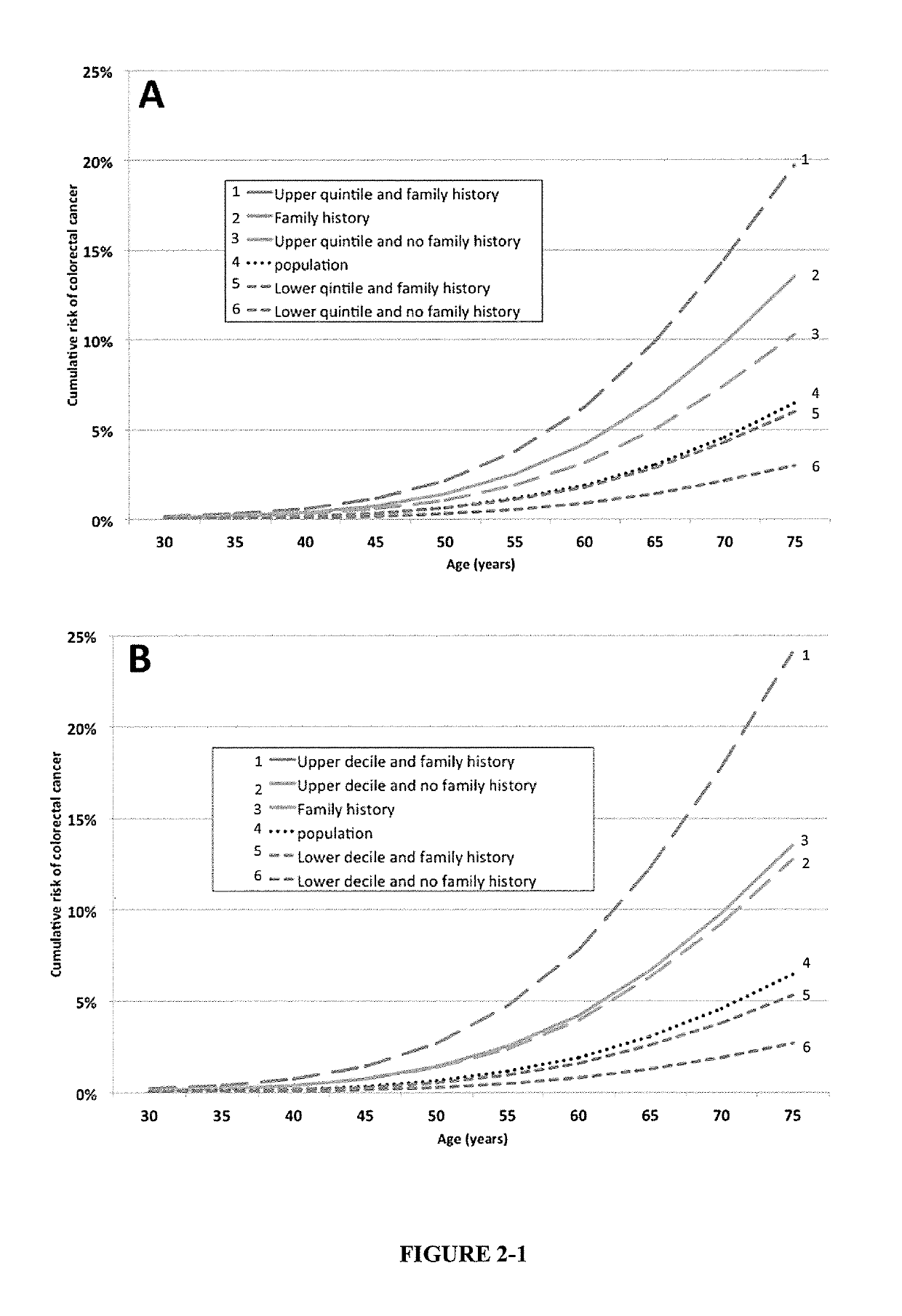
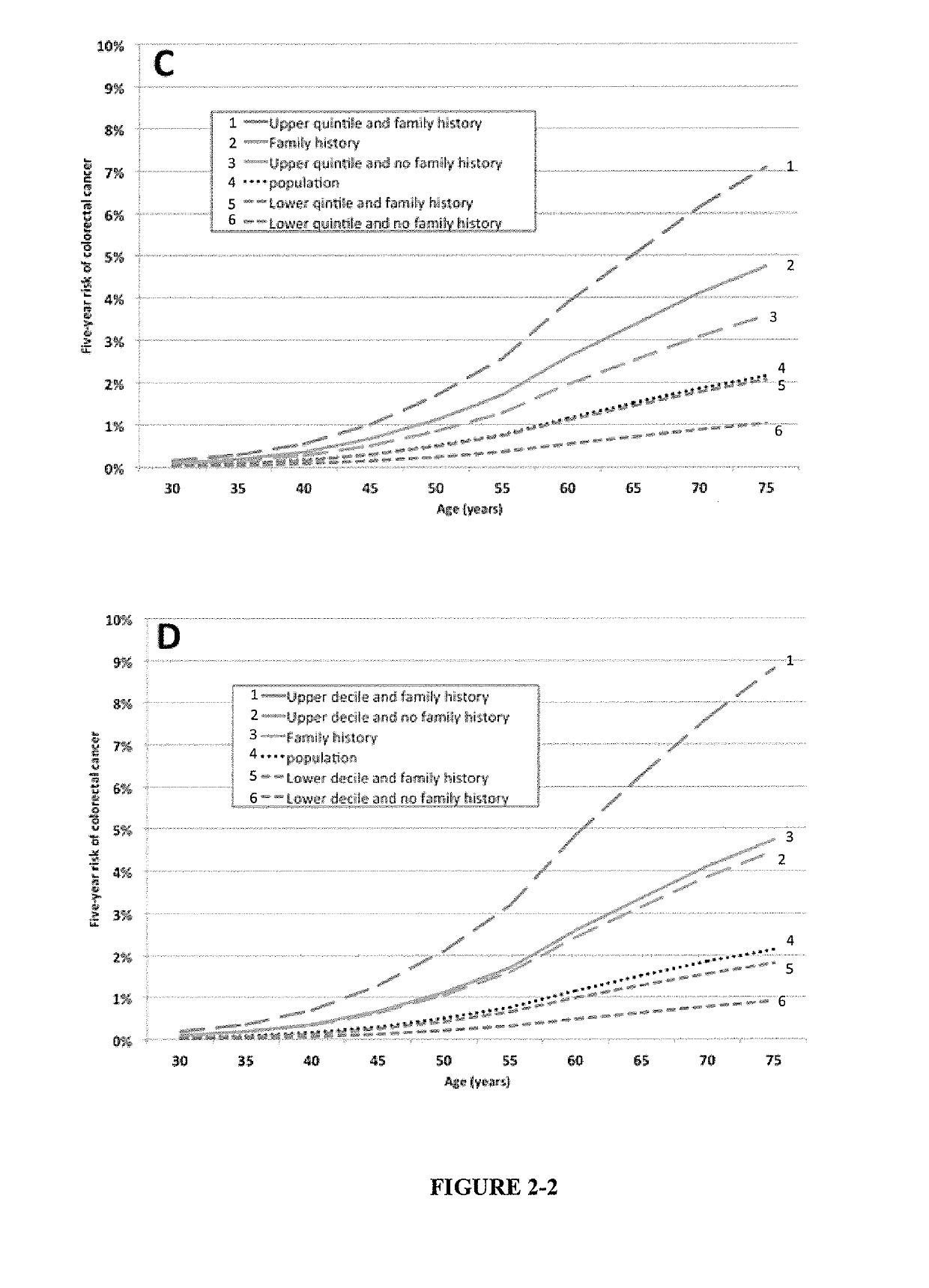
The present disclosure relates to methods and systems for assessing the risk of a human subject for developing colorectal cancer. These methods may be combined with the subjects clinical risk to improve risk analysis. Such methods may be used to assist decision making about appropriate colorectal cancer screening regimens.
Owner:UNIVERSITY OF MELBOURNE
Kit for detecting bacteria DNAs in faeces and application thereof in colorectal cancer diagnosis
The invention discloses a kit for detecting bacteria DNAs in faeces and the application thereof in colorectal cancer screening, diagnosis or auxiliary diagnosis. Bacteria DNAs in faeces are extracted and sequenced to obtain the species and abundance of bacteria, and colorectal cancer diagnosis is conducted based on the abundance of various bacteria. Compared with existing noninvasive colorectal cancer screen methods which have already been used clinically or for patent application, the kit has the advantages that the kit is completely noninvasive, colorectal cancer can be predicted and diagnosed more accurately, and the ROC area under the curve can be as high as 0.994.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Colorectal cancer screening kit based on excrement sample
PendingCN112210601AMicrobiological testing/measurementMicroorganism based processesDNA methylationOncology
The invention discloses a method and a kit capable of accurately detecting colorectal cancer or colorectal cancer precancerous lesion adenoma as well as a related non-transient computer readable medium and a computer system. According to the scheme provided by the invention, the blood components, the gene mutation, the DNA methylation and the intestinal bacteria content in the excrement sample ofa subject are comprehensively analyzed from at least four dimensions, so that early detection of the colorectal cancer, particularly accurate detection in the adenoma stage, is realized.
Owner:SINGLERA GENOMICS (SHANGHAI) LTD
DNA methylation marker for early colorectal cancer and adenoma, method for detecting DNA methylation marker and application of DNA methylation marker
PendingCN113278693AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationCpG siteA-DNA
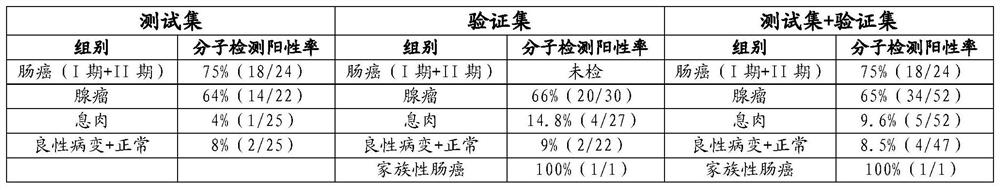
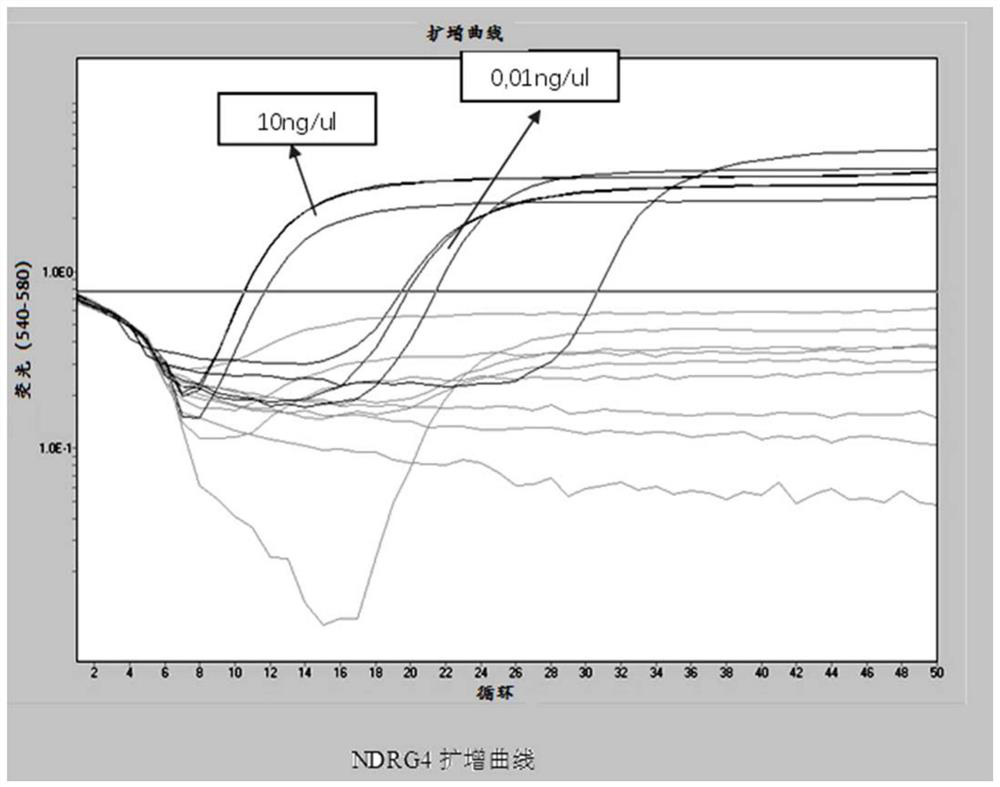
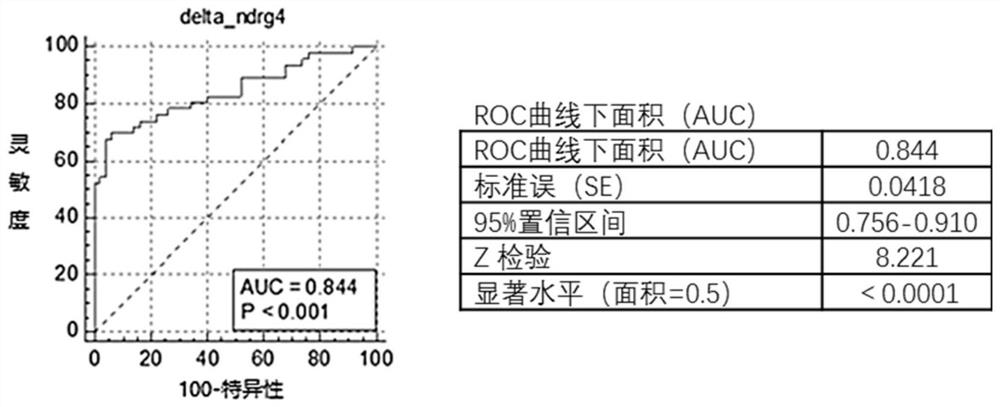
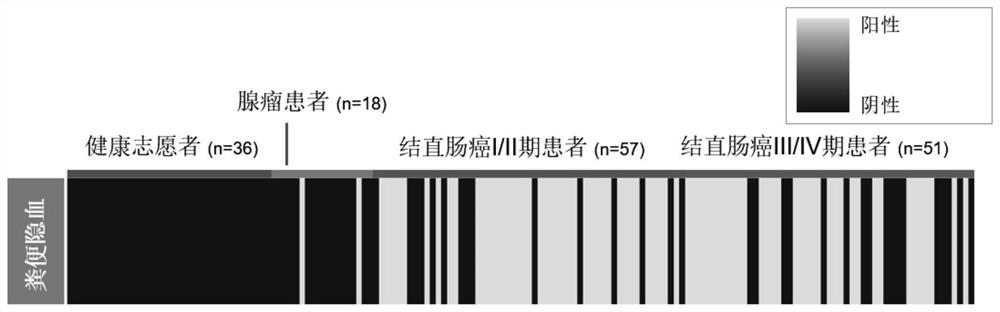
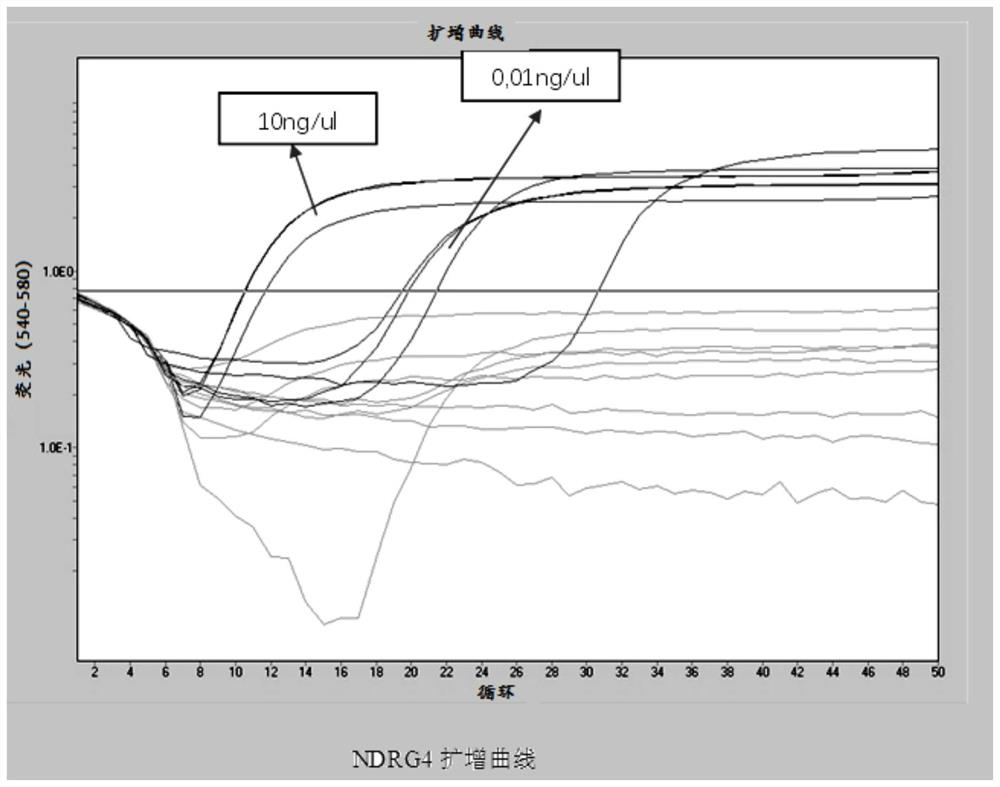
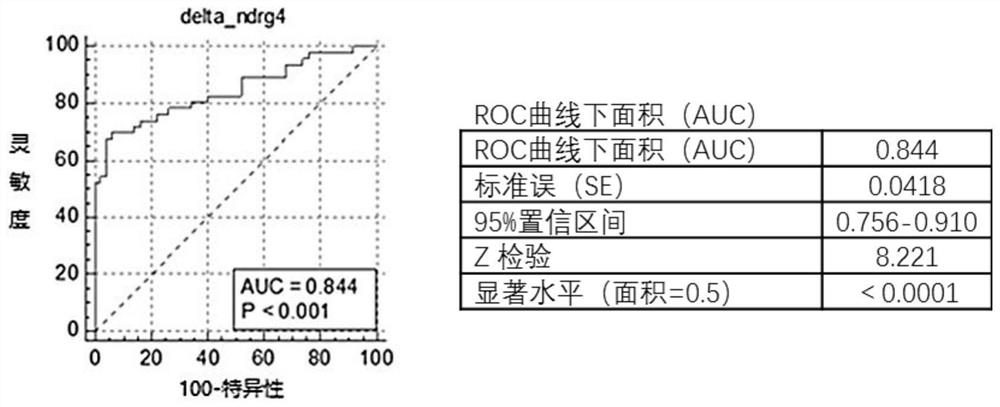
The invention provides a DNA methylation marker for diagnosis, screening and risk prediction of early colorectal cancer and adenoma. The marker is characterized in that a CpG site in an NDRG4 gene is simultaneously methylated on at least two sites of the 38th site, the 47th site, the 50th site and the 52nd site in SEQ ID NO: 1. The invention also provides a method, a primer pair, a probe and a kit for detecting the DNA methylation marker. The method provided by the invention has good specificity and high sensitivity on early colorectal cancer and adenoma, is low in detection cost and simple to operate, and is beneficial to wide application of early colorectal cancer screening.
Owner:SHANGHAI GENECHEM CLINICAL LAB INC
Colorectal cancer screening method based on excrement sample
ActiveCN112210602AHealth-index calculationMicrobiological testing/measurementDNA methylationOncology
The invention discloses a method and a kit capable of accurately detecting colorectal cancer or colorectal cancer precancerous lesion adenoma as well as a related non-transient computer readable medium and a computer system. According to the scheme provided by the invention, the blood components, the gene mutation, the DNA methylation and the intestinal bacteria content in the excrement sample ofthe subject are comprehensively analyzed from at least four dimensions, so that early detection of the colorectal cancer, particularly accurate detection in the adenoma stage, is realized.
Owner:SINGLERA GENOMICS (SHANGHAI) LTD
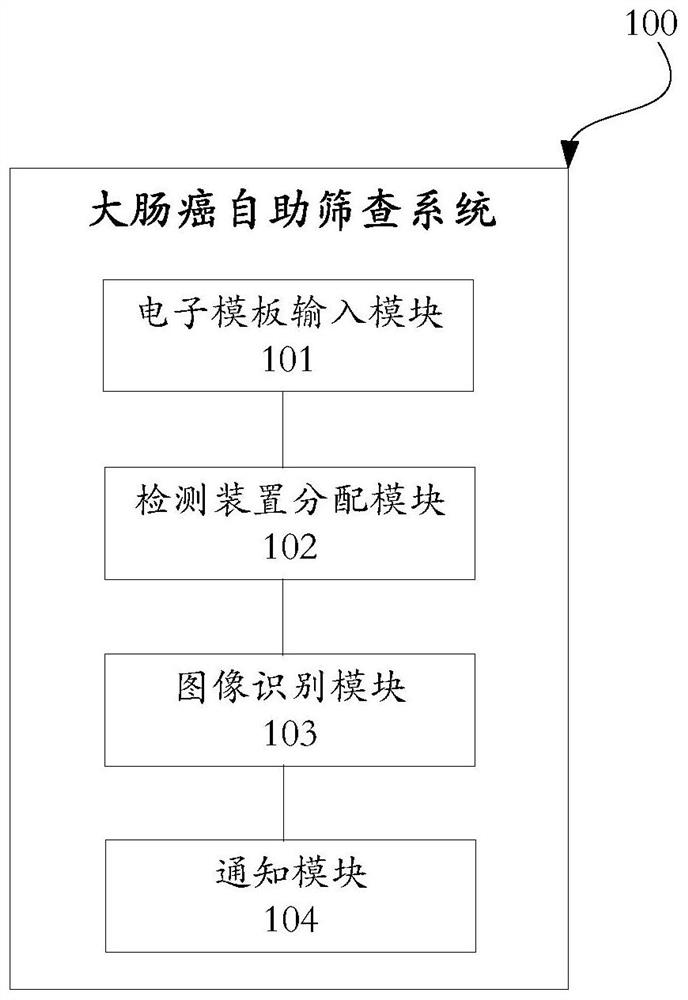
Colorectal cancer self-service screening system and method based on image recognition, terminal and medium
PendingCN114429804AImprove accuracyImage analysisHealthcare resources and facilitiesDiseaseDiseases history
The invention provides a colorectal cancer self-service screening system and method based on image recognition, a terminal and a medium, and provides an information filling electronic template for a screening object to fill in an informed agreement, a risk assessment questionnaire, personal information and related symptoms and disease histories on line; according to dwelling address information in the personal information, a screening service mechanism is correspondingly allocated to the screening object for the screening object to get the fecal occult blood detection device with the ID number, and the ID number of the fecal occult blood detection device is associated with the personal information of the corresponding screening object; and reading and identifying a detection result image of the fecal occult blood detection device to obtain a colorectal cancer screening result of a screening object associated with the fecal occult blood detection device. A screening object can complete signing of an informed agreement, filling of a risk assessment questionnaire, distribution of a screening service mechanism, reservation receiving of a fecal occult blood detection device, online interpretation of a fecal occult blood result, feedback of a preliminary screening result, diagnostic enteroscopy notification and the like in a self-service manner, and the screening accuracy and convenience are greatly improved.
Owner:SHANGHAI MUNICIPAL CENT FOR DISEASE CONTROL & PREVENTION
Application of serum protein marker combination to colorectal cancer screening, diagnosis and treatment
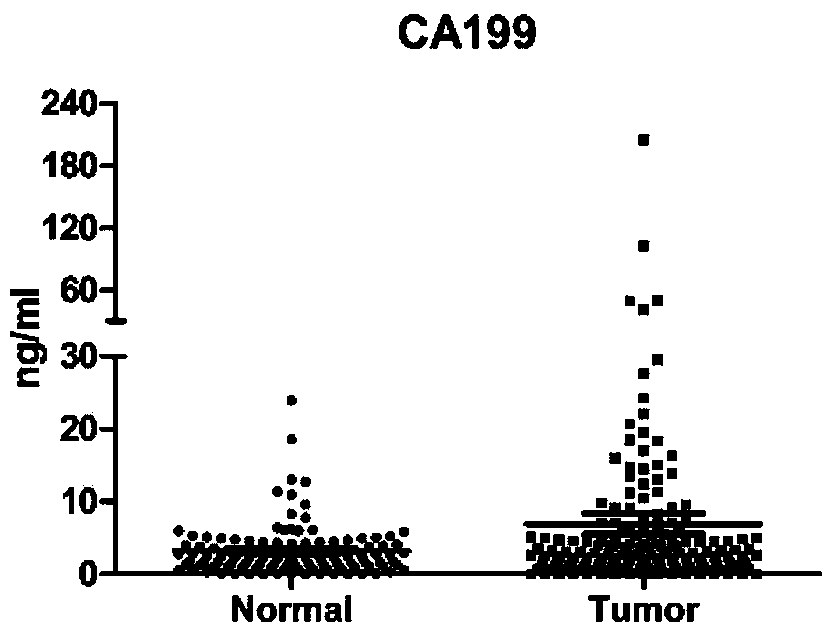
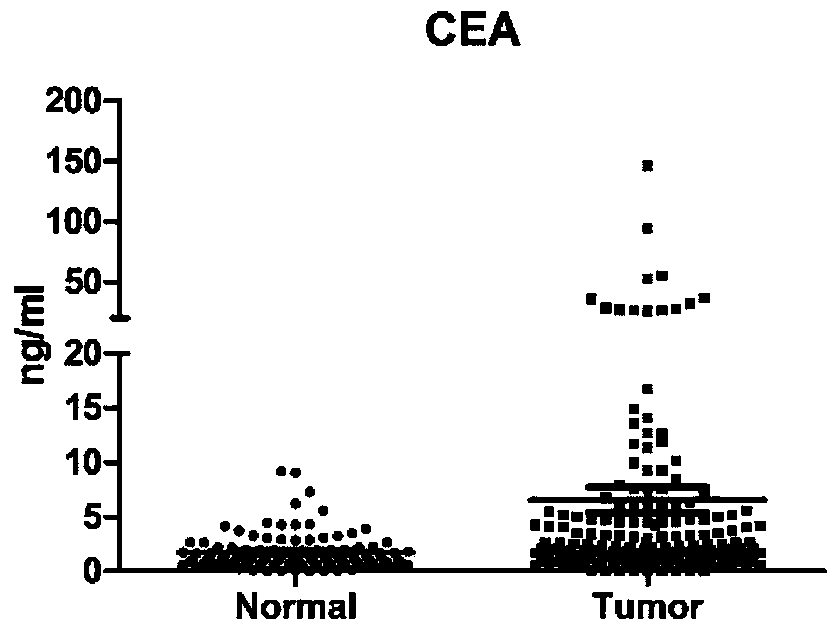
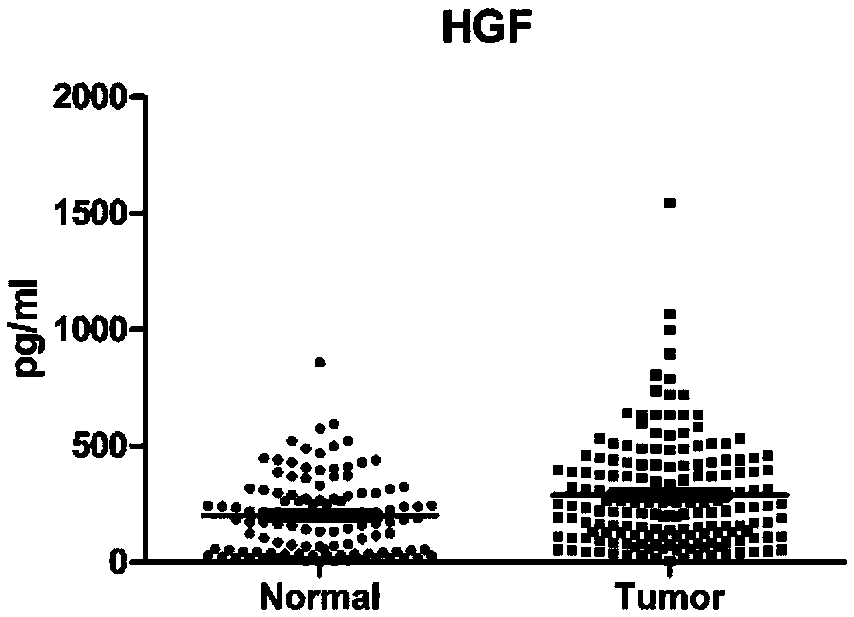
The invention belongs to the technical field of molecular biology and clinical detection, and relates to a protein combination, wherein the protein combination is selected from any two or three or four or five or six of CA19-9, CEA, HGF, IP-10, MIP-1beta and SDF-1alpha. The invention further relates to a ligand combination specifically bound with proteins in the protein combination, and application of the protein combination or the ligand combination to preparation of a kit for colorectal cancer screening, auxiliary diagnosis, treatment monitoring and prognosis judging. A serum protein markercombination can be used for colorectal cancer screening, auxiliary diagnosis, treatment monitoring and prognosis judging and particularly used for early colorectal cancer screening and auxiliary diagnosis, and the sensitivity of the serum protein marker combination is superior to that of a single protein marker and commonly used clinical serum markers CA19-9 and CEA in detecting.
Owner:CANCER INST & HOSPITAL CHINESE ACADEMY OF MEDICAL SCI
Tumor gene diagnosis marker combined with carcinoembryonic antigen and application of tumor gene diagnosis marker
PendingCN113817824AHigh screening accuracyMicrobiological testing/measurementMaterial analysisCarcinoembryonic Antigen PositiveOncology
Owner:NANJING FANYIDA BIOTECHNOLOGY CO LTD
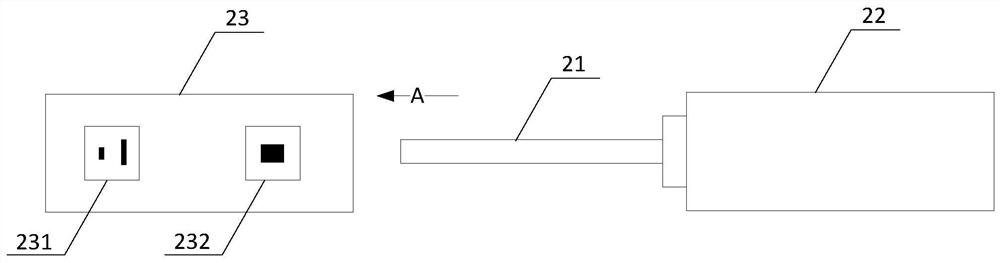
Fecal occult blood detection device
The invention provides a detection device for fecal occult blood, which is composed of a main casing, a sampling stick, a water discharge valve, an upper cover, a lower cover, and a test paper holder. The device integrates the functions of fecal sample collection, dissolution, chromatography, and test strip detection. It can complete sample collection and immunoassay fecal occult blood detection at one time. Self-check. The device is easy to carry and operate, low in cost, simplifies the detection process, does not need to open fecal liquid in the whole process, and can realize independent detection by the tester, which reduces the burden on medical staff, improves the screening rate of colorectal cancer, and reduces the risk of colorectal cancer. morbidity and mortality.
Owner:SHANGHAI REALBIO TECH CO LTD
Colorectal cancer screening examination and early detection method
PendingUS20220214345A1Contributes immensely to the global burden of cancersReduce incidenceDisease diagnosisDiseaseBiomarker panel
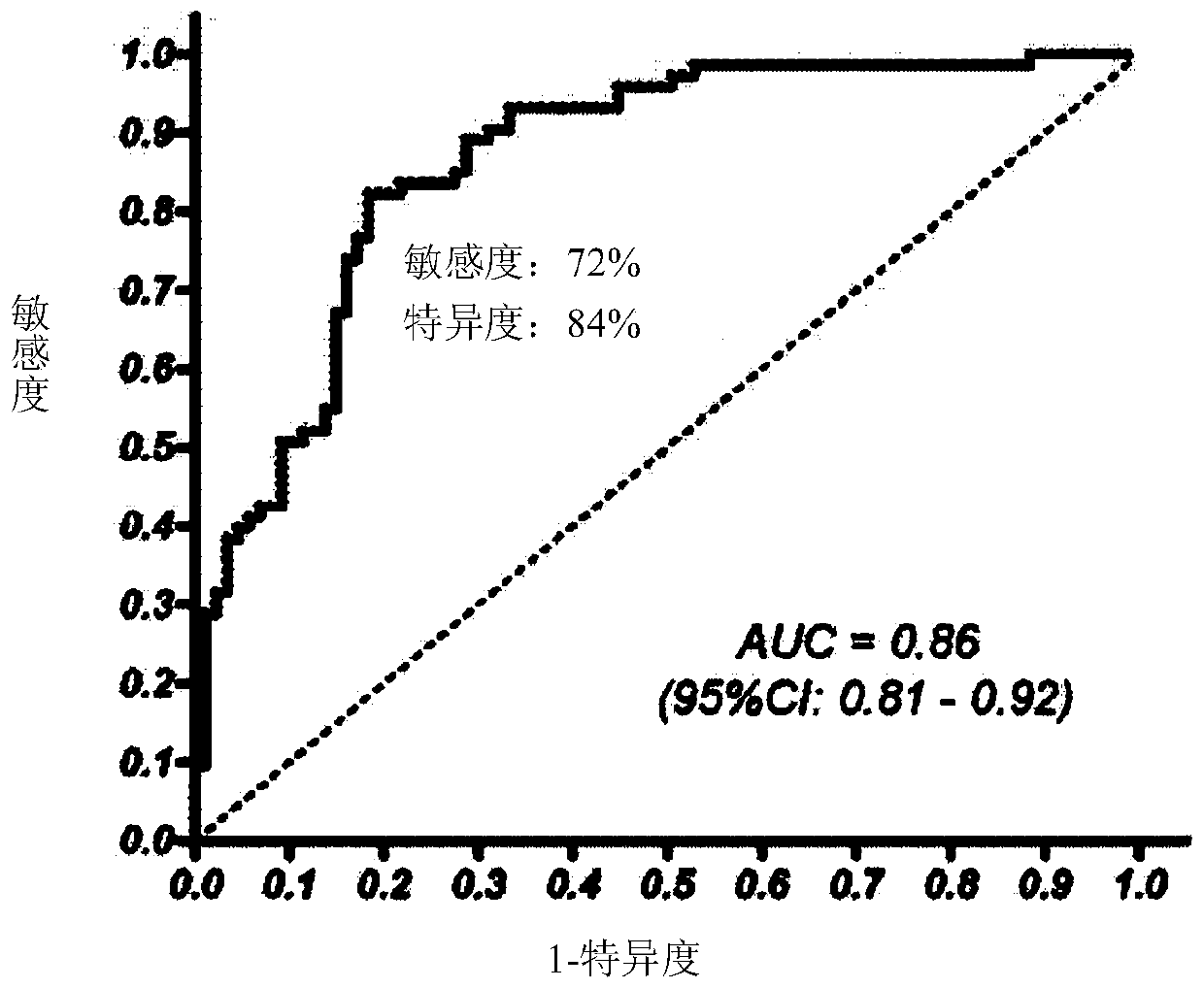
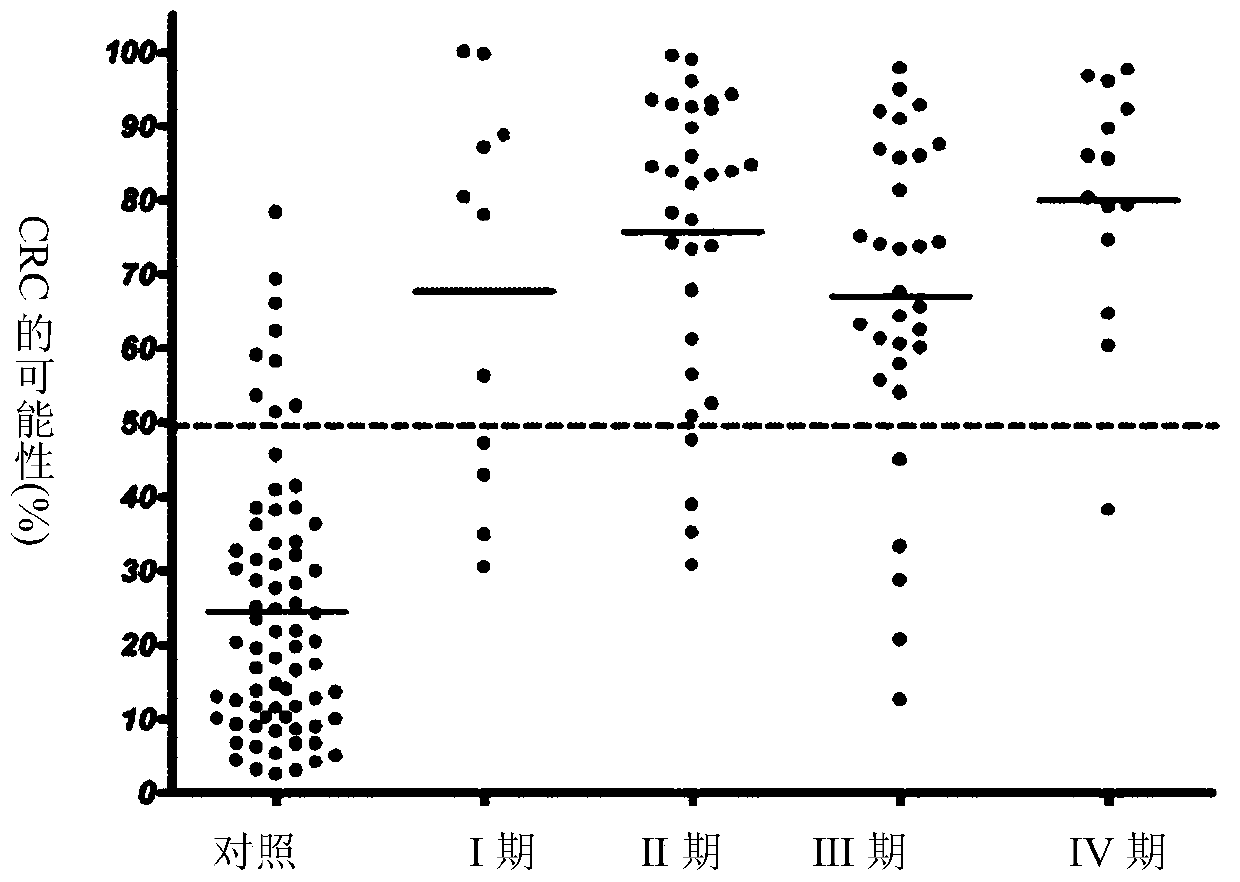
The present invention pertains to a new method for the diagnosis, prognosis, stratification and / or monitoring of a therapy, of cancer, preferably colorectal cancer (CRC), in a subject. The method is based on the determination of the level of a panel of least one, preferably 3, 4 and most preferably at least 5, protein biomarker selected from the group consisting of the protein biomarkers Amphiregulin (AREG), Carcinoembryonic antigen (CEA), Insulin like growth factor binding protein 2 (IGFBP2), Keratin, type I cytoskeletal 19 (KRT19), Mannan binding lectin serine protease 1 (MASP1), Osteopontin (OPN), Serum paraoxonase lactonase 3 (PON3) and Transferrin receptor protein 1 (TR), in the biological sample obtained from the subject. The new biomarker panel of the invention allows diagnosing and even stratifying various cancer diseases. Furthermore, provided are diagnostic kits for performing the non-invasive methods of the invention. Since the biomarker panel of the invention provides a statistically robust method independent of the protein detection technology used, and considering that the biomarker panel of the invention is detected in plasma samples of the subjects, the invention provides an early detection screening examination that may be applied to a larger population.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
Kit for detecting DNA methylation markers of early colorectal cancer and adenoma
PendingCN113699237AStrong specificityHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationCpG siteA-DNA
The invention provides a DNA methylation marker for diagnosis, screening and risk prediction of early colorectal cancer and adenoma. The marker is characterized in that a CpG site in an NDRG4 gene is simultaneously methylated at least two sites of the 38th site, the 47th site, the 50th site and the 52th site in SEQ ID NO.:1. The invention also provides a method, a primer pair, a probe and a kit for detecting the DNA methylation marker. The method provided by the invention has good specificity and high sensitivity on early colorectal cancer and adenoma, is low in detection cost and simple to operate, and is beneficial to wide application of early colorectal cancer screening.
Owner:SHANGHAI GENECHEM CLINICAL LAB INC
Methionine aminopeptidase overexpression in the peripheral blood and peripheral blood mononuclear cells is a marker for colorectal cancer screening, diagnosis and prognosis
ActiveUS11506663B2Health-index calculationMicrobiological testing/measurementPeripheral blood mononuclear cellMethionine aminopeptidase
Owner:VASTCON
Automatic sampling auxiliary device for colorectal cancer screening
PendingCN114295855ARealize automatic sampling functionVersatileBiological testingMechanical engineeringElectrical and Electronics engineering
The invention discloses an automatic sample injection auxiliary device for colorectal cancer screening, which comprises a vibration disc and a material conveying rail matched with the vibration disc for feeding, the material conveying rail horizontally extends left and right, one end of the material conveying rail back to the vibration disc is provided with a material inserting platform, and a moving and inserting mechanism is arranged between the material inserting platform and the material conveying rail; a baffle is arranged at the tail end of the material conveying rail, and a visual detection device is arranged above the tail end of the material conveying rail. A first linear module for controlling the material inserting platform to horizontally move left and right and a second linear module for controlling the material inserting platform to horizontally move front and back are arranged at the bottom of the material inserting platform, the first linear module is arranged on a sliding table of the second linear module, and the material inserting platform is arranged on a sliding table of the first linear module; the device further comprises a mechanical arm. The automatic sample injection auxiliary device has the characteristics of convenience in sample injection and rapidness in testing, and is complete in overall function and high in practicability.
Owner:嘉兴康华医学检验有限公司
A kind of colorectal cancer screening kit
ActiveCN105907859BImprove complianceEasy to operateMicrobiological testing/measurementDisease diagnosisColon rectal cancerOncology
The invention discloses a colorectal cancer screening kit, which comprises related reagents for detecting expression level of any one or more of 18 genes provided by the invention. The invention also discloses an application of 18 genes in preparation of a colorectal cancer screening reagent. The invention further discloses a detection method of 18 genes, a detection kit and a colorectal cancer screening method. The kit disclosed by the invention can accurately detect whether a sample to be detected gets colorectal cancer, and has a good prospect in clinical application.
Owner:BIOMERIEUX SA
Methods for assessing risk of developing colorectal cancer
The present disclosure relates to methods and systems for assessing the risk of a human subject for developing colorectal cancer. These methods may be combined with the subjects clinical risk to improve risk analysis. Such methods may be used to assist decision making about appropriate colorectal cancer screening regimens.
Owner:UNIVERSITY OF MELBOURNE
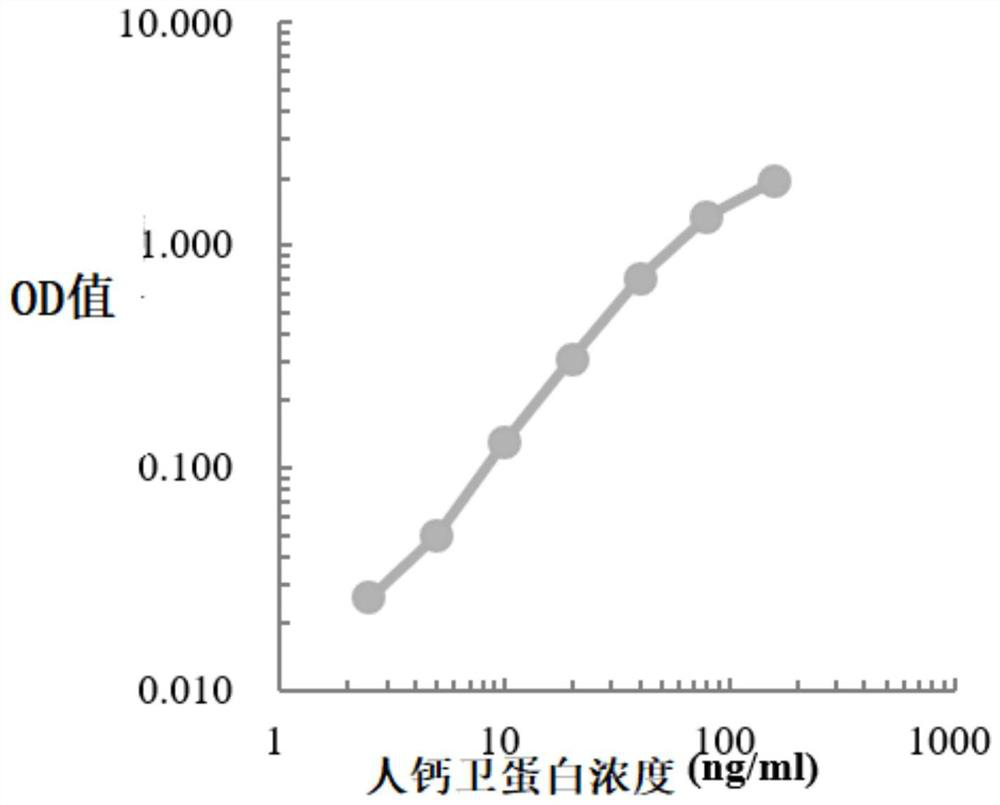
Colorectal cancer excrement protein biomarker as well as kit and application thereof
PendingCN112526138AConducive to screeningImprove accuracyChemiluminescene/bioluminescenceMaterial analysis by electric/magnetic meansColon rectal cancerBiologic marker
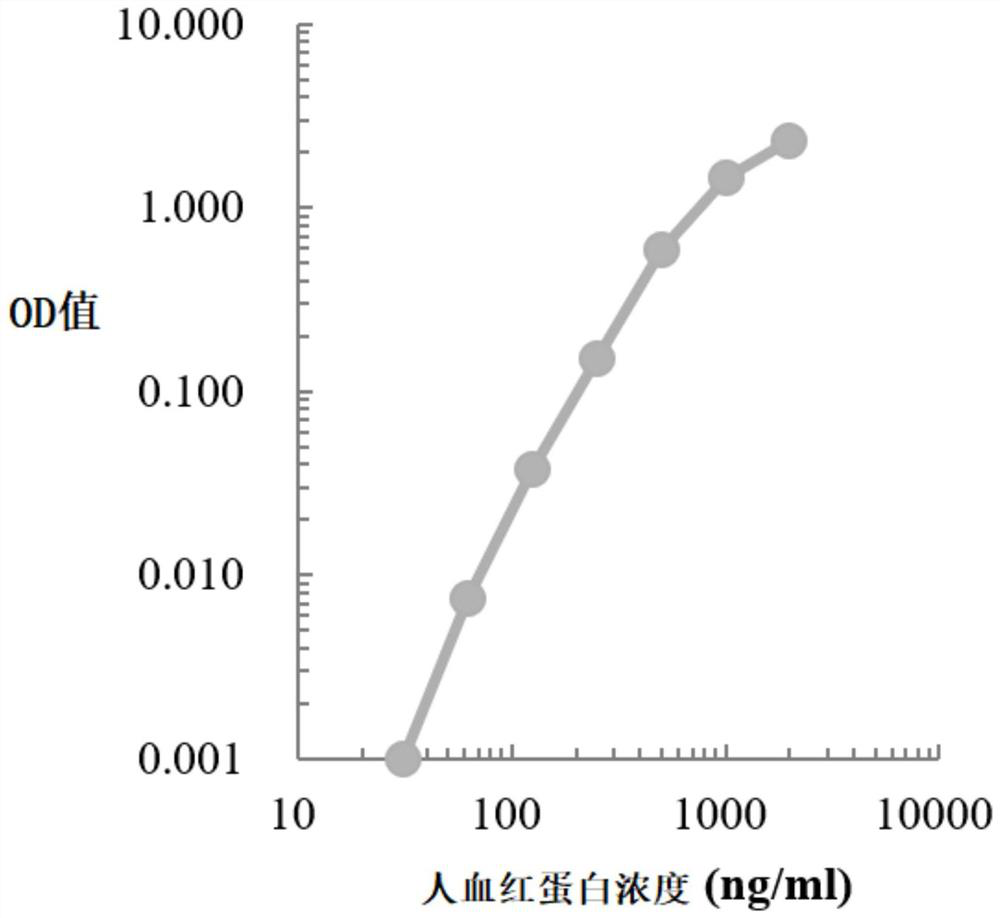
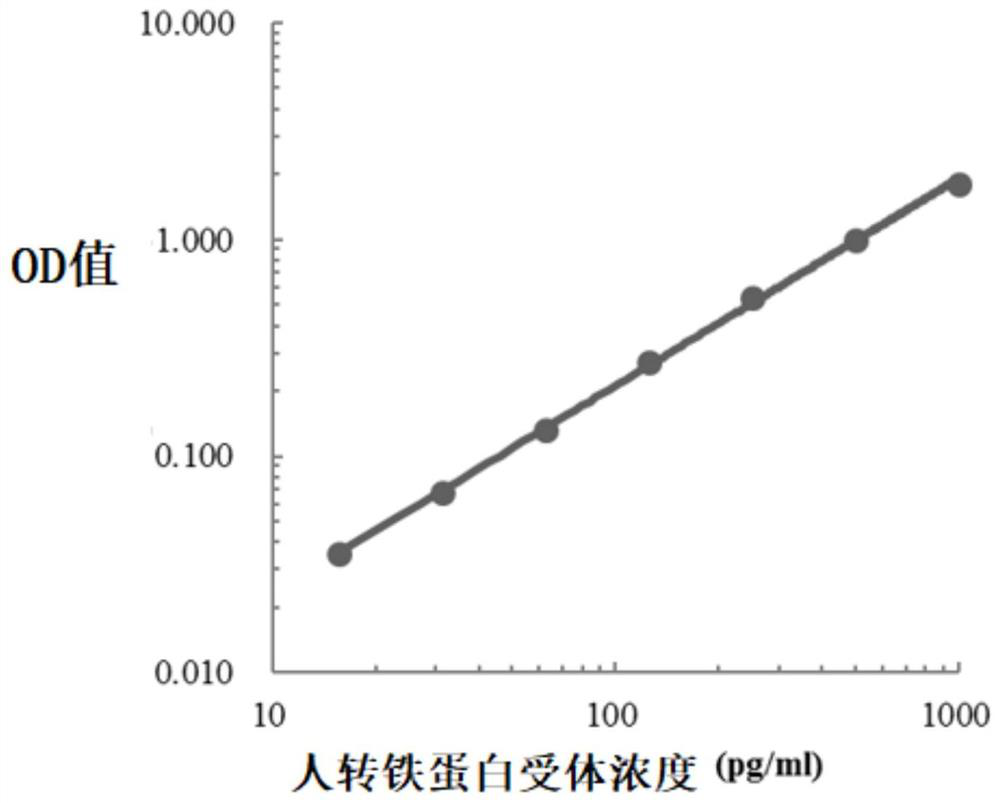
The invention relates to the field of immunodetection and particularly relates to a colorectal cancer excrement protein biomarker and a kit and application thereof. The biomarker comprises at least atransferrin receptor. It is found for the first time that the transferrin receptor can be used for fecal screening of colorectal cancer, the colorectal cancer screening sensitivity and the negative and positive coincidence rate can be remarkably improved by means of the biomarker containing the transferrin receptor, rapid and convenient colorectal cancer preliminary screening is achieved, the colorectal mirror efficiency is improved, and colorectal cancer screening cost is reduced; the price can be received by the public, the detection time is short, and the method is suitable for POCT and household detection and has wide application value.
Owner:张春明 +1
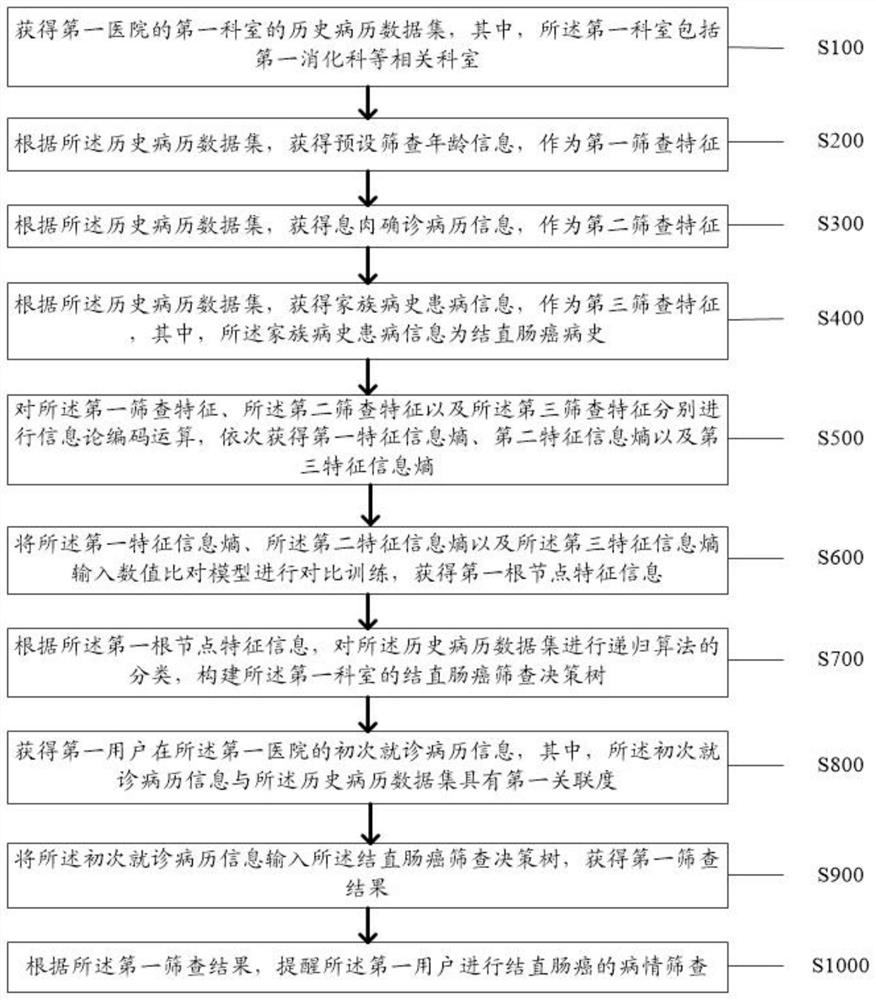
Colorectal cancer risk screening method and system based on decision tree
PendingCN114334131AScreening features are accurateScreening science is accurate and fastMedical data miningMedical automated diagnosisMedical recordHistory disease
The invention discloses a colorectal cancer risk screening method and system based on a decision tree, and the method comprises the steps: obtaining a historical medical record data set of a first department; taking a preset screening age as a first screening feature, taking a polyp definite medical record as a second screening feature, and taking a family medical history disease as a third screening feature; obtaining a first feature information entropy, a second feature information entropy and a third feature information entropy in sequence through information theory coding operation; inputting the first root node feature into a numerical value comparison model to obtain a first root node feature; constructing a colorectal cancer screening decision tree; obtaining an initial medical record of the first user; inputting the image into a colorectal cancer screening decision tree to obtain a first screening result; and reminding the first user to screen the condition of the colorectal cancer. The technical problems that in the prior art, based on the disease characteristics of the colorectal cancer, massive user medical records cannot be efficiently and rapidly screened, potential hazards of the colorectal cancer cannot be found as early as possible, and user treatment is delayed are solved.
Owner:THE FIRST MEDICAL CENT CHINESE PLA GENERAL HOSPITAL
A kit for screening colorectal cancer using common small molecules
The invention discloses a kit for screening colorectal cancer by using common small molecules, and belongs to the field of biotechnology. The kit adopts common small molecules C16:1-OH, Na, C5DC / C16, C10:2 / C10, Glu, Lys, Trp and C4 / C3 as colorectal cancer diagnosis molecular markers, molecule detection models corresponding to the common small molecules are established, and the sensitivity and specificity of colorectal cancer detection are respectively 90.9% and 100%. By adopting the indexes, a remarkable differentiation effect for colorectal cancer and normal references can be achieved, the screening process is accurate, rapid, simple and convenient, and the kit is prior to markers which are used in recent years to study colorectal cancer.
Owner:大连润生康泰医学检验实验室有限公司 +1
Novel stool-based protein biomarkers for colorectal cancer screening
PendingUS20200408763A1Increase the number ofImprove accuracyMaterial analysisBiologic markerCancer research
The invention relates to methods for typing a sample of an individual suffering from a colorectal cancer, or suspected of suffering therefrom. A preferred sample is stool. The invention further relates to methods for determining a level of expression of at least two extracted protein expression molecules in stool, based on the quantified reaction products, and to methods of assigning treatment to an individual that was typed as suffering from colorectal cancer according to the invention.
Owner:STICHTING HET NEDERLANDS KANKER INST ANTONI VAN LEEUWENHOEK ZIEKENHUIS +1
A colorectal cancer screening marker composition, its selection method, and colorectal cancer screening kit
ActiveCN113817836BReduce mortalityReduce harmMicrobiological testing/measurementDNA/RNA fragmentationWhite blood cellOncology
Owner:南京求臻基因科技有限公司
A kind of colorectal cancer screening kit
ActiveCN103667437BImprove complianceEasy to operateMicrobiological testing/measurementDisease diagnosisMedicineGene
The invention discloses a colorectal cancer screening kit, which comprises related reagents for detecting expression level of any one or more of 18 genes provided by the invention. The invention also discloses an application of 18 genes in preparation of a colorectal cancer screening reagent. The invention further discloses a detection method of 18 genes, a detection kit and a colorectal cancer screening method. The kit disclosed by the invention can accurately detect whether a sample to be detected gets colorectal cancer, and has a good prospect in clinical application.
Owner:BIOMERIEUX SA
Biomarker for mass colorectal cancer screening
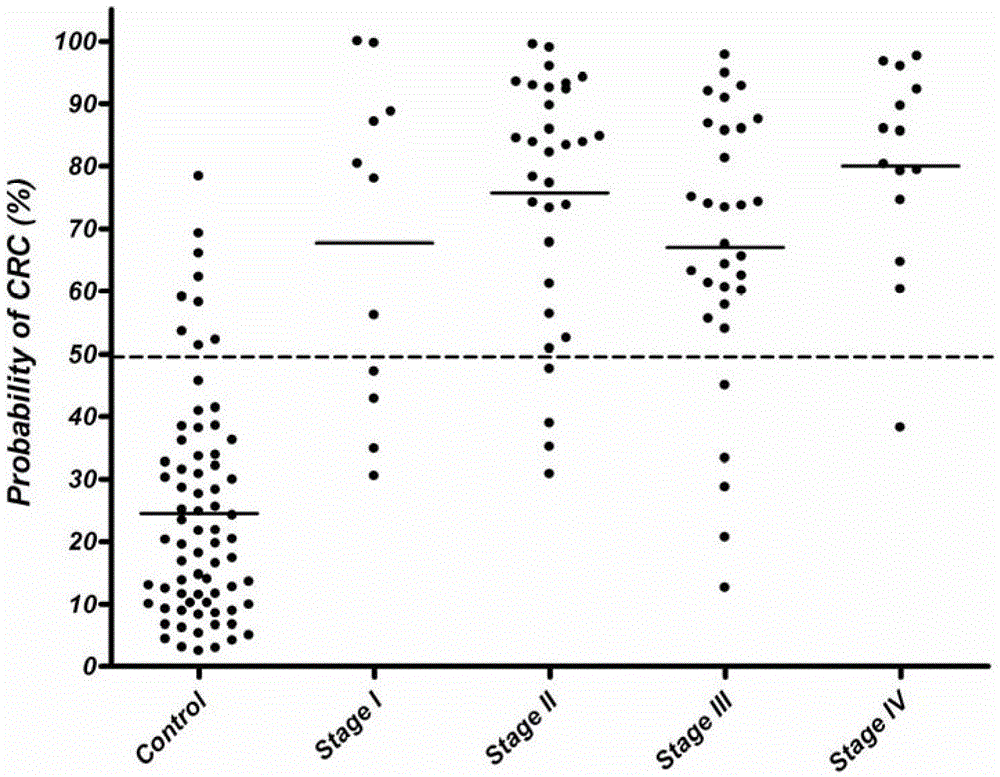
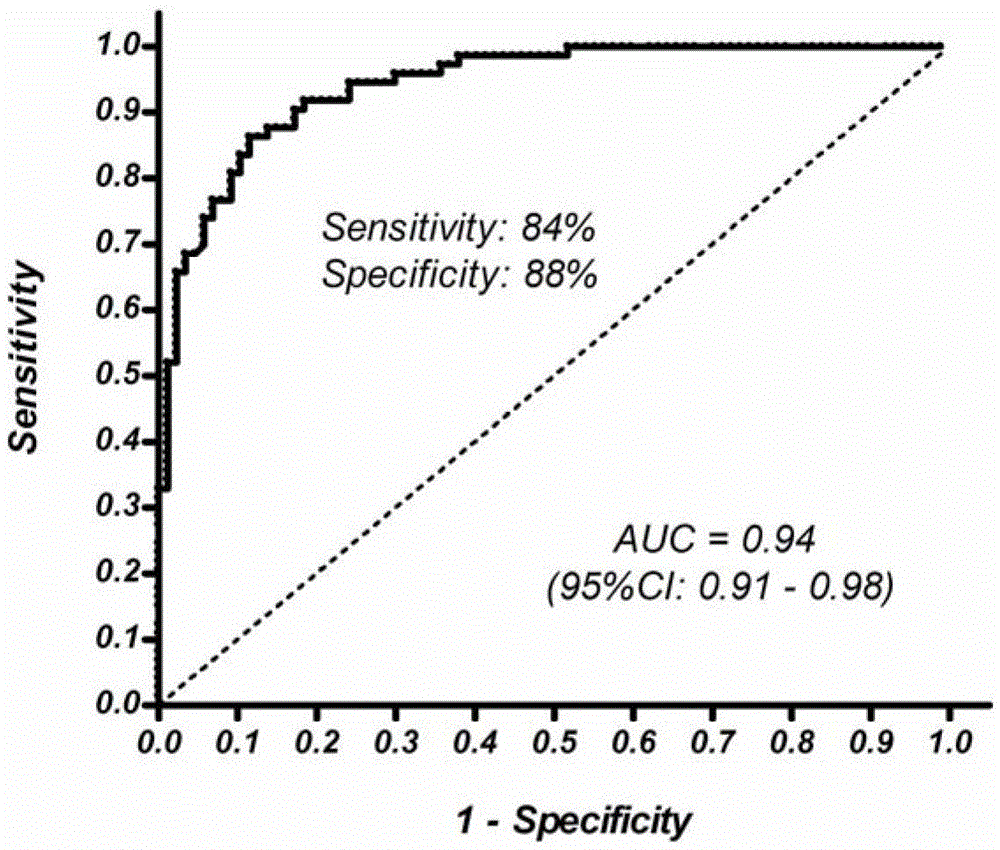
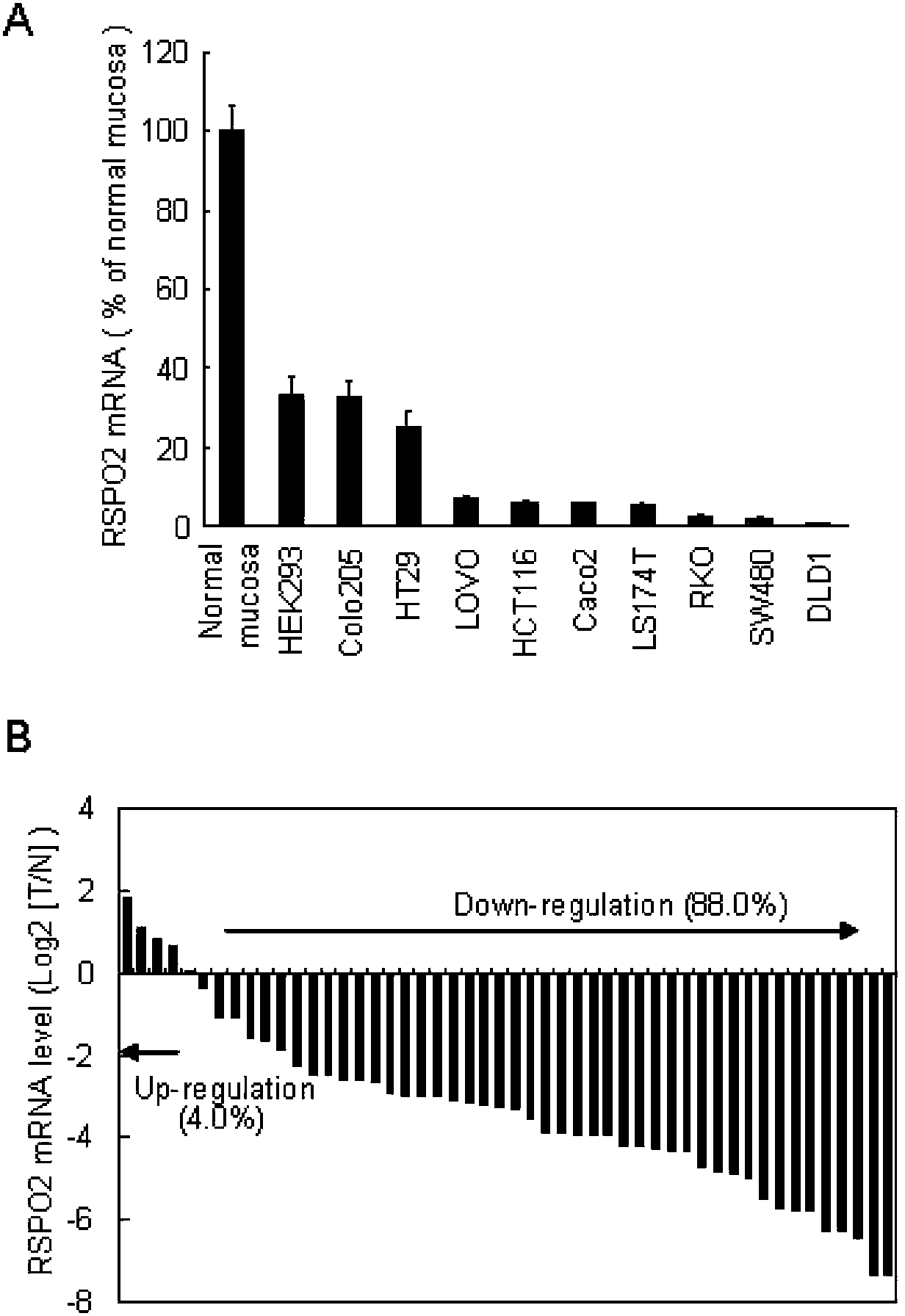
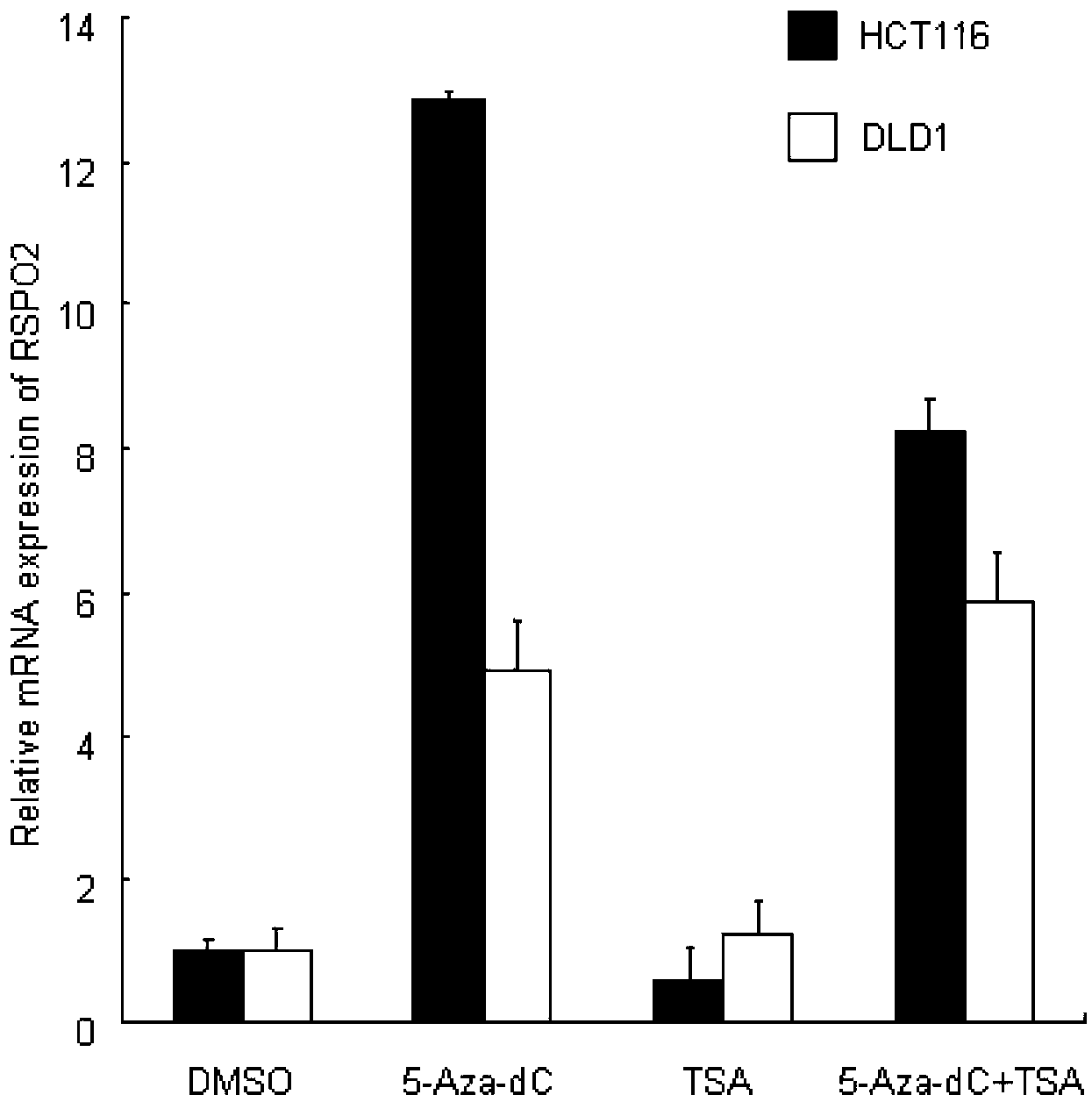
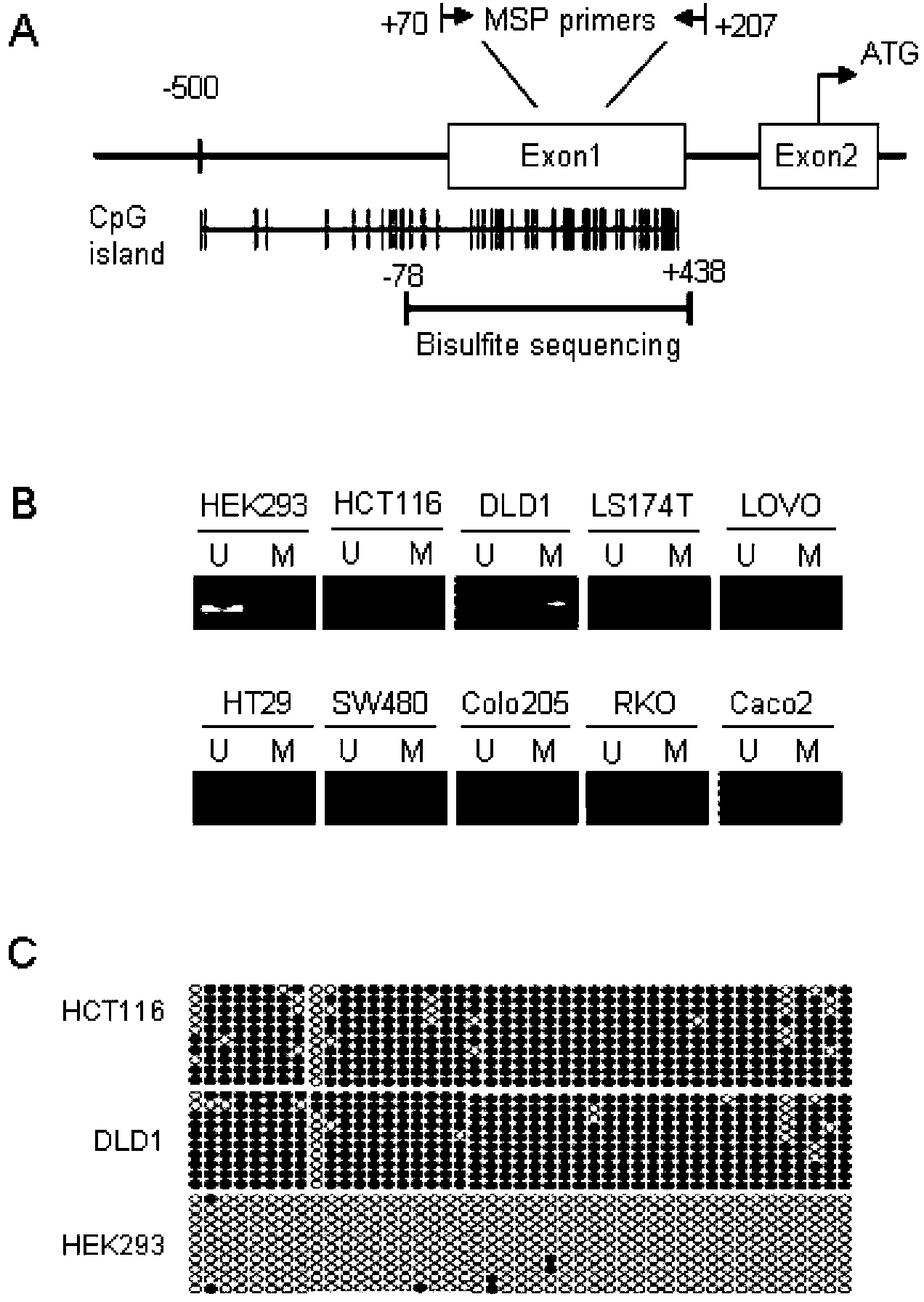
The present invention discloses a biomarker for mass colorectal cancer screening, wherein the biomarker is RSPO2 gene. The present invention further discloses a RSPO2 gene methylation MSP detection method, which comprises the following steps: extracting genome DNA containing RSPO2 gene, and detecting purity and content; carrying out treatment modification by using sodium bisulfite; adopting the modified DNA as a template, designing RSPO2 methylation specific primers, carrying out PCR amplification, and then taking the PCR product to carry out electrophoresis detection; and analyzing a result, wherein a methylation positive result exists if a 139 bp band product is amplified through the RSPO2 methylation specific primers, ie., RSPO2 methylation exists. According to the present invention, RSPO2 is adopted as a biomarker for mass colorectal cancer screening, and an RSPO2 methylation status detection method is provided, such that whether patients suffer from colorectal cancer can be detected, and characteristics of no invasion, convenient and flexible sample collection, and the like are provided.
Owner:WENZHOU MEDICAL UNIV
Excrement occult blood detection device
ActiveCN112014553ARealize autonomous detectionReduce the burden onBiological testingOncologyTest strips
The invention provides an excrement occult blood detection device which is composed of a main shell, a sampling rod, a water drain valve, an upper cover, a lower cover and a test paper rack. The device integrates the functions of excrement sample collection, dissolution, chromatography, test strip detection and the like, can finish sample collection and immune excrement occult blood detection at one time, and is suitable for a detector to perform excrement occult blood self-service detection at any place and at any time in families, hospitals and the like. The device is easy and convenient tocarry and operate and low in cost, the detection process is simplified, excrement liquid does not need to be opened in the whole process, self-detection of a detector can be achieved, the burden of medical staff is relieved, the colorectal cancer screening rate is increased, and the occurrence rate and the death rate of colorectal cancer are reduced.
Owner:SHANGHAI REALBIO TECH CO LTD
Marker and probe composition for colorectal cancer screening and application of marker and probe composition
PendingCN114395628ASensitive and specific detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationAsymptomaticOncology
The invention discloses a marker and a probe composition for colorectal cancer screening and application of the marker and the probe composition. The marker is selected from any one of 28 markers. According to the present invention, with the application of the marker, the methylation state of the gene can be sensitively and specifically detected so as to be used for the detection of the peripheral blood free DNA, and the composition is used for the screening of the asymptomatic population in the non-invasive manner so as to reduce the harm caused by the invasive detection; the composition has higher sensitivity and accuracy, and can realize real-time monitoring.
Owner:BIOCHAIN BEIJING SCI & TECH
Detection of colorectal cancer
The present disclosure provides, inter alia, methods for colorectal cancer screening and compositions related thereto. In various embodiments, the present disclosure provides methods for colorectal cancer screening comprising analyzing the methylation status of one or more methylation biomarkers, as well as compositions related thereto. In various embodiments, the present disclosure provides a method for colorectal cancer screening that includes screening for the methylation status of one or more methylation biomarkers in cfDNA, e.g., in ctDNA. In various embodiments, the present disclosure provides a method for colorectal cancer screening, comprising screening the methylation status of one or more methylation biomarkers in cfDNA, e.g., in ctDNA, using MSRE-qPCR.
Owner:UNIVERSAL DIAGNOSTICS SL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com