Colorectal cancer excrement protein biomarker as well as kit and application thereof
A biomarker and colorectal cancer technology, applied in the field of immunoassay, can solve the problems of long detection period, unsatisfactory effect, and low response to promotion, and achieve the goal of improving sensitivity and accuracy, fast and convenient screening, and wide application value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1ELISA kit
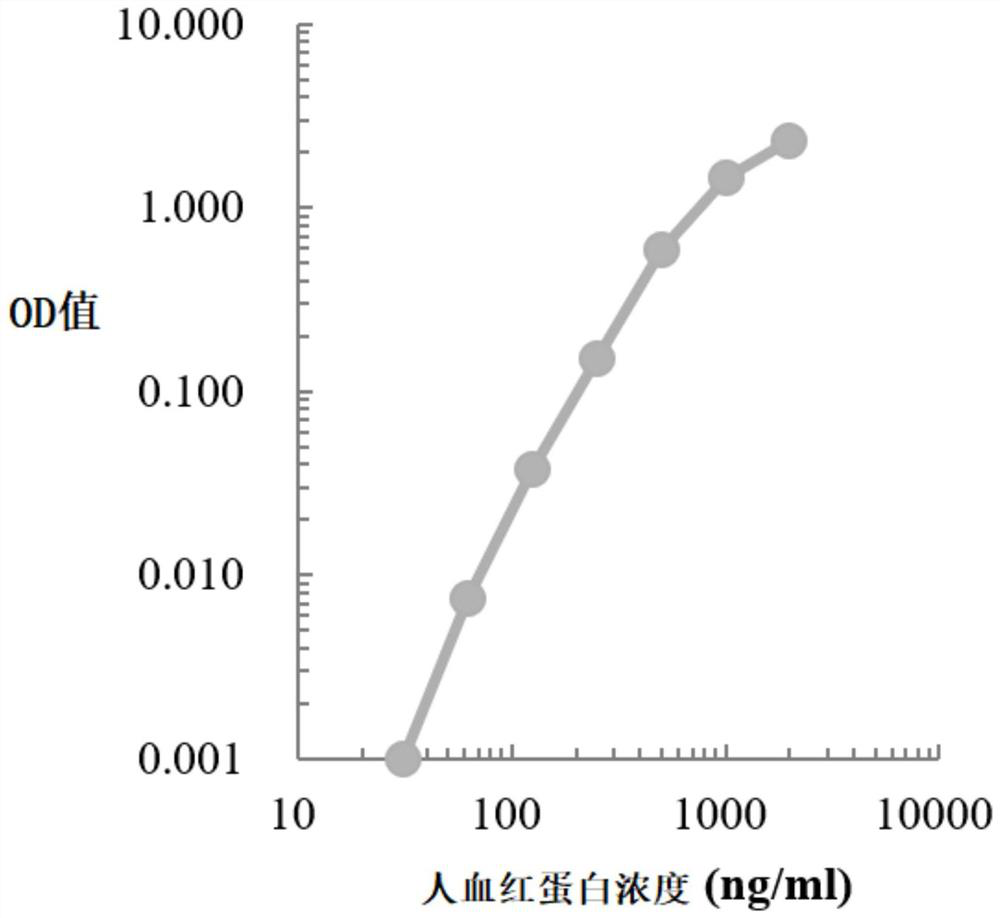
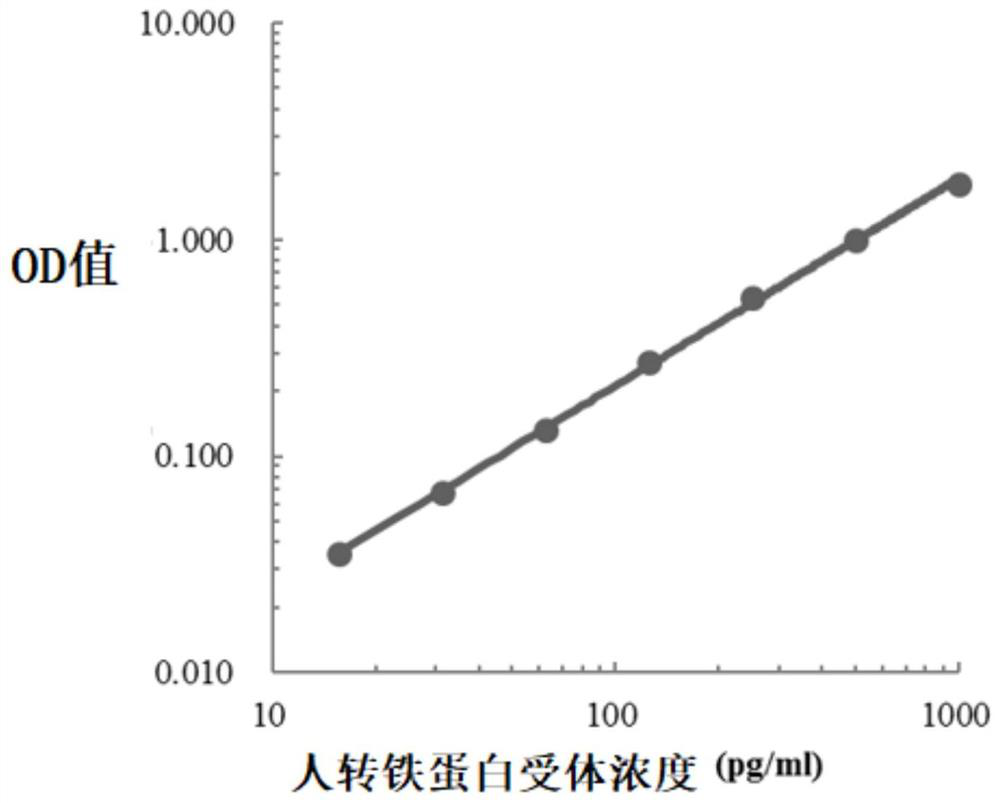
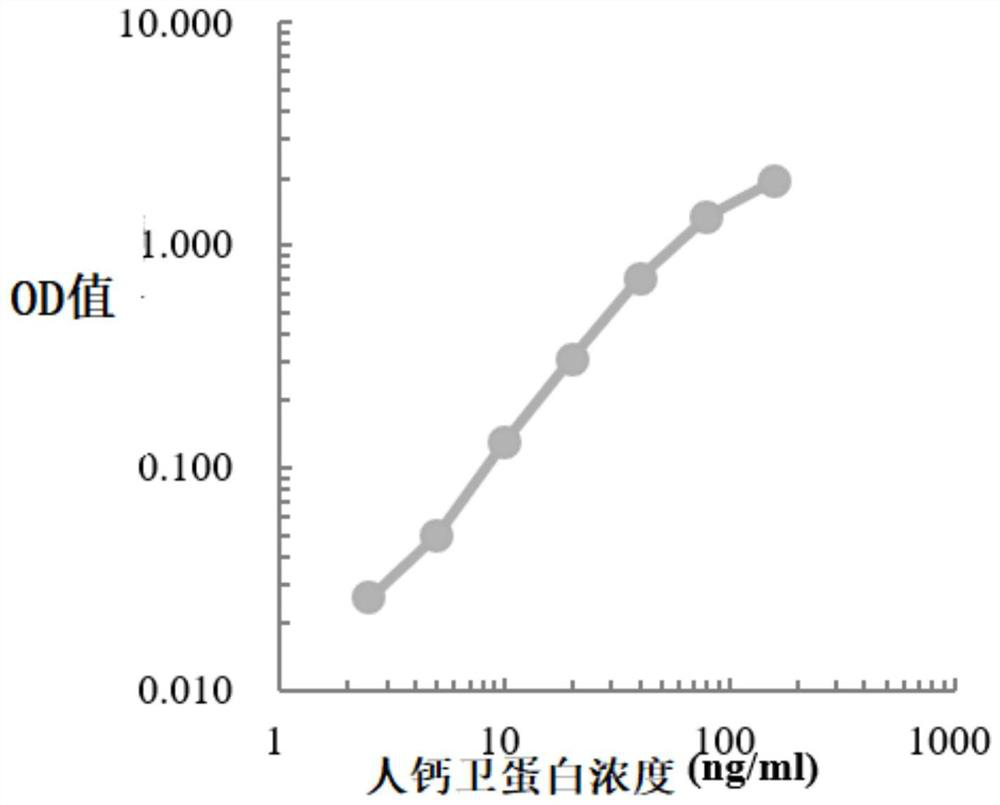
[0044] In this example, the classic double-antibody sandwich ELISA method was used to construct the standard curve of the indicators to be tested (paired antibodies and standard products were from American R&D systems, Sigma, Beijing Yiqiao Shenzhou and Finland Medix, etc.), human hemoglobin, human transferrin The standard curves of receptor and human calprotectin were as follows Figure 1-Figure 3 shown.
Embodiment 2
[0045] Detection of colorectal cancer fecal protein biomarkers in the sample of Example 2
[0046] In this example, the kit described in Example 1 is used to detect the concentration of colorectal cancer fecal protein biomarkers in the sample, which specifically includes the following steps:
[0047] 1. Sample collection and processing:
[0048] Collect stool samples from patients with colorectal cancer and healthy people. Samples from patients with colorectal cancer are collected before surgery to avoid mixing with urine. Take 1-2g of feces into a collection tube and send them to the laboratory for processing within 4 hours (directly extracted or placed at -80 degrees) Save and process together after the samples are collected); see Table 1 and Table 2 for specific information.
[0049] Table 1 Tumor sample information (Fudan University Cancer Hospital)
[0050] 28 samples age 40-89 gender 19 males / 9 females tumor site rectal can...
Embodiment 3
[0072] The first people's hospital of Zhangjiagang city affiliated to Soochow University and the fifth hospital of Shenyang city collected outpatients of general surgery and gastroenterology (excluding women's menstrual cycle, taking aspirin, clopidogrel and other anticoagulant drugs; colonoscopy not due to tumor obstruction) back to the blind). The natural defecation sample before taking the bowel cleanser, about 1-2g, is collected within 2-3 hours and stored in a -20°C refrigerator, and transferred to a -80°C refrigerator within 2 weeks for storage, and the sample is taken according to the method described in Example 2 , Lysis, and ELISA detection, colonoscopy and pathological results as the standard, and at the same time use the lysed supernatant for occult blood detection; calculate the sensitivity and specificity of the combined index (combination of hemoglobin, TFR and calprotectin), and compare it with the existing occult blood method, hemoglobin quantitative method for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com