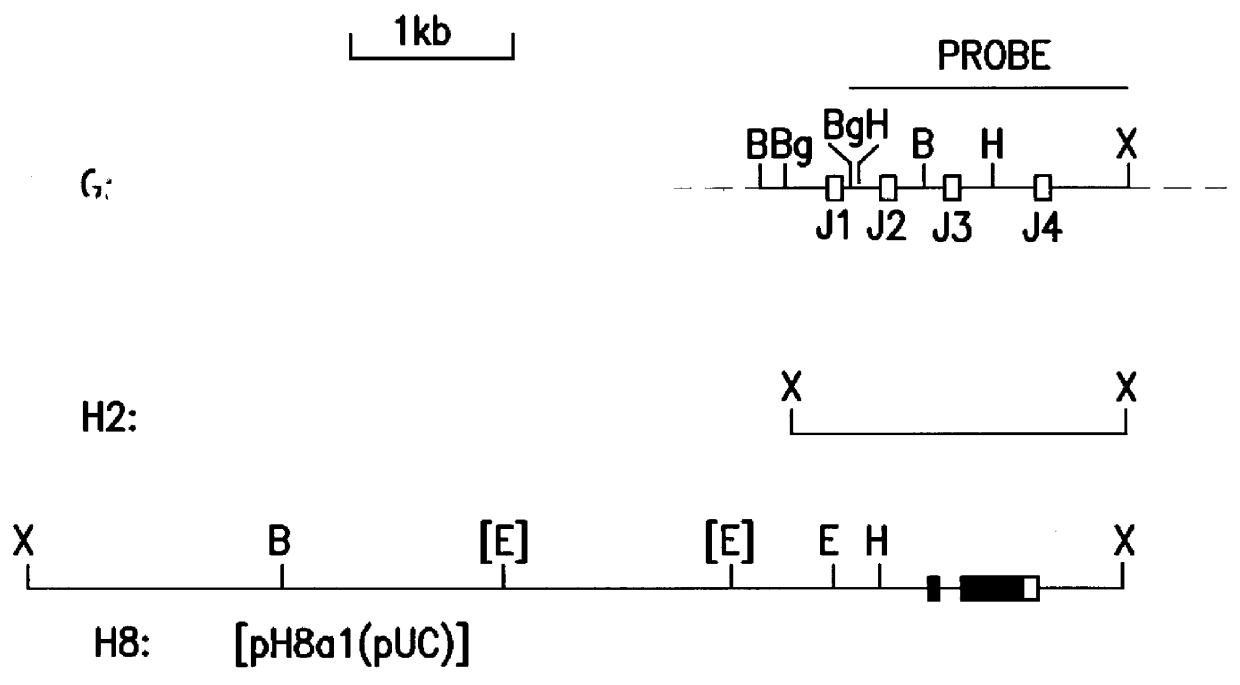
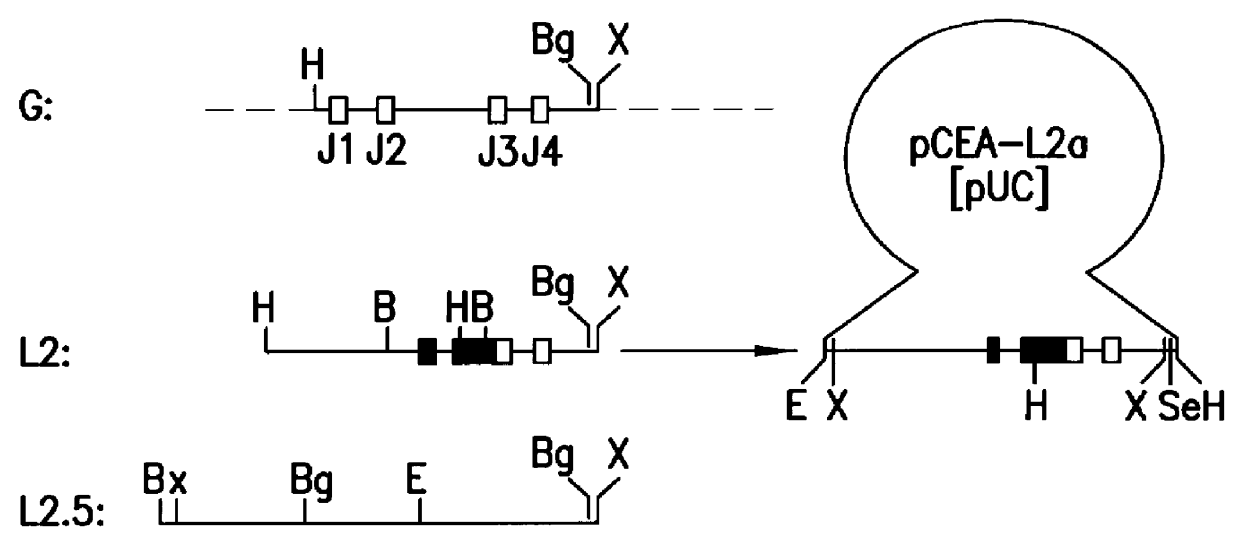
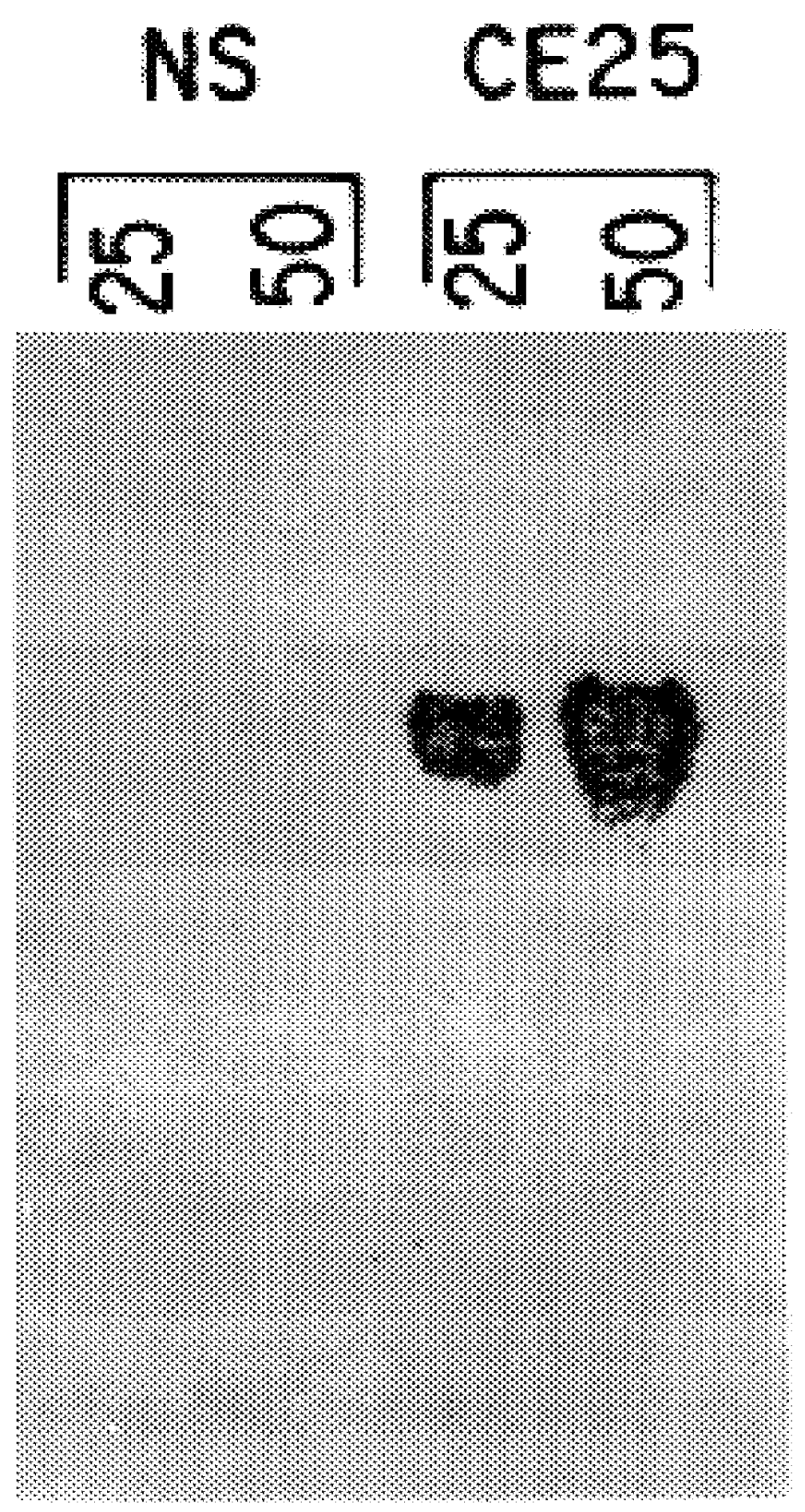
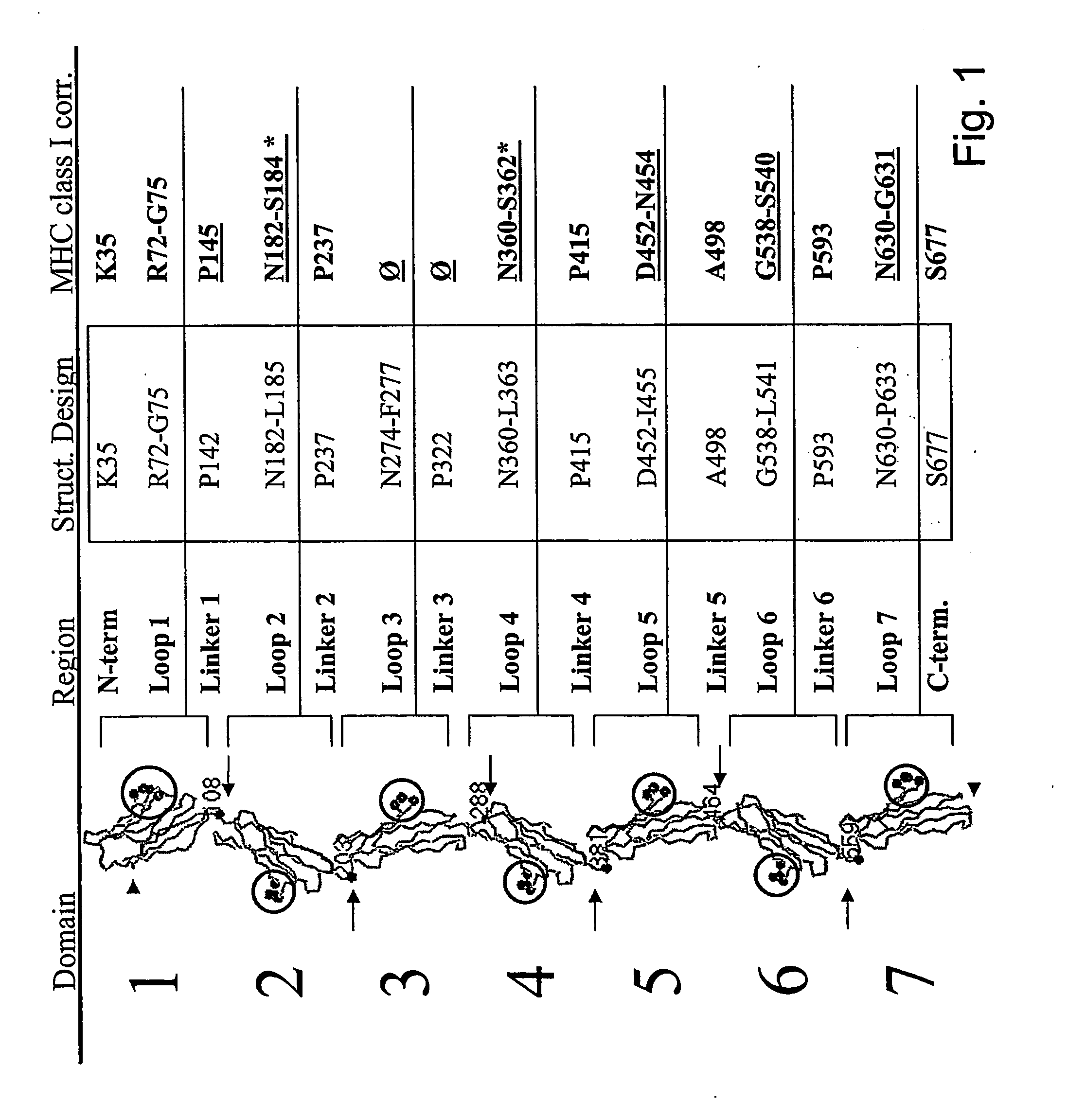
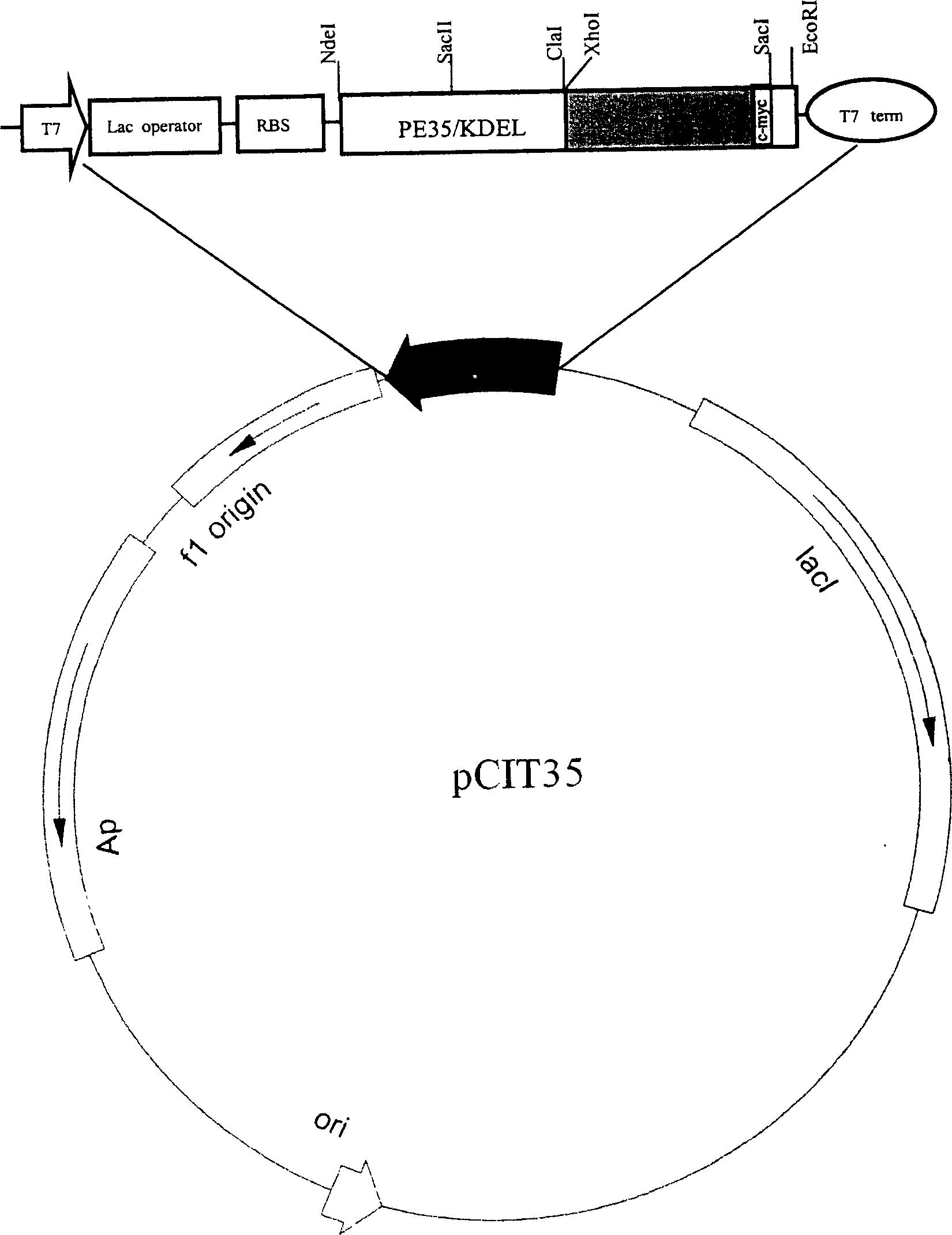
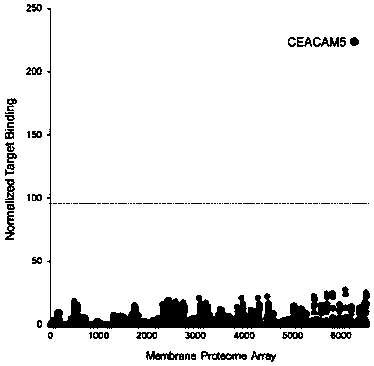
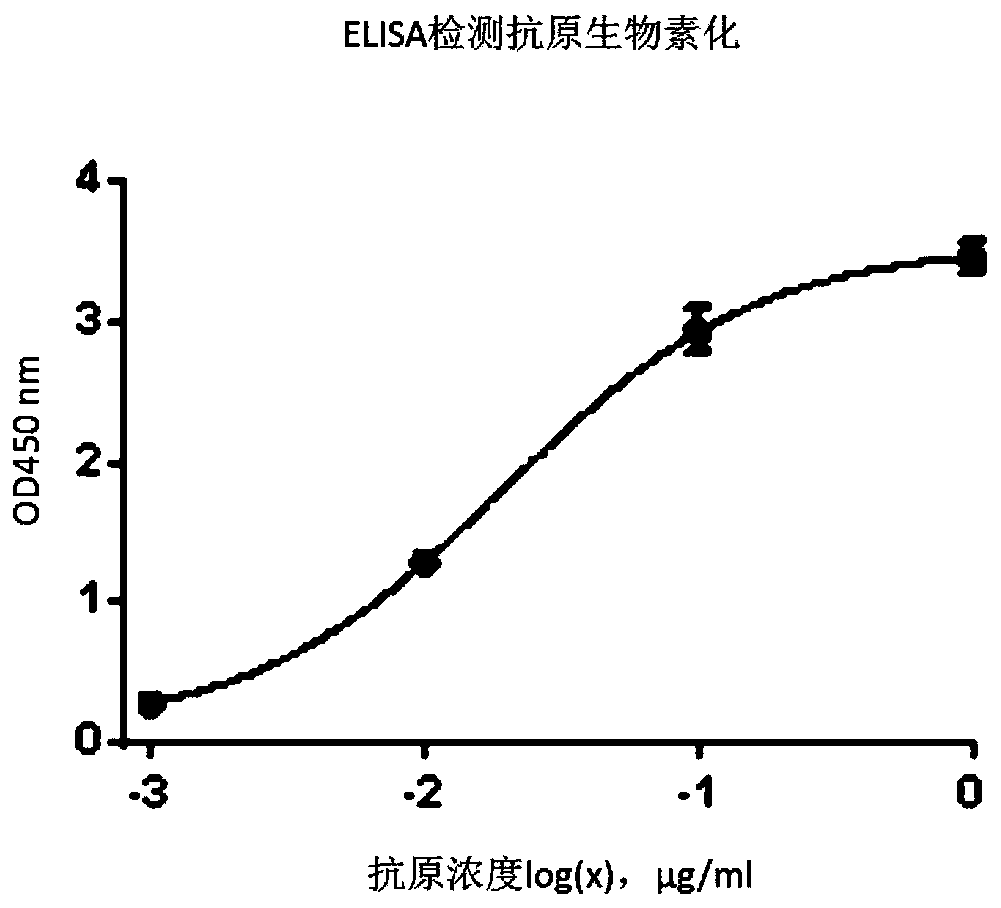
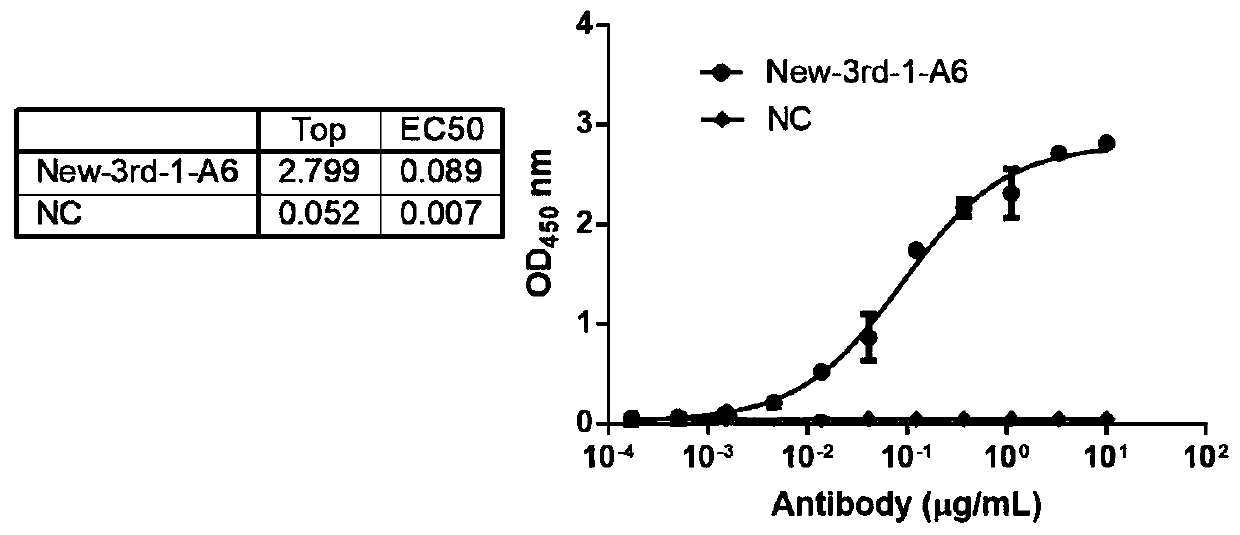
Patents
Literature
57 results about "Carcinoembryonic Antigen Positive" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
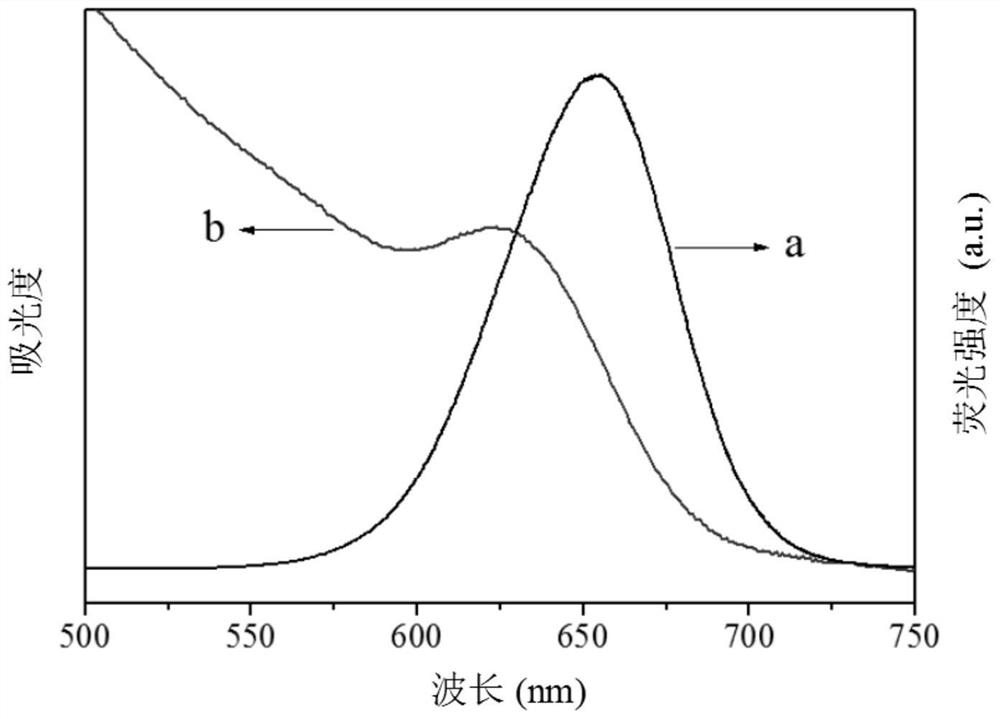
A laboratory test result indicating the presence of carcinoembryonic antigen in a sample.
Chimeric antibodies
InactiveUS6020153ALow percentage and absence of bindingAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainIn vivo
The invention relates to murine / human chimeric monoclonal antibodies with high specificity to and affinity for human carcinoembryonic antigen (CEA), derivatives thereof, processes for the preparation of these antibodies and their derivatives, DNAs coding for heavy and light chains of these antibodies, processes for the preparation of said DNAs, mammalian cell lines that produce and secrete the antibodies and processes for the preparation of said cell lines. The chimeric antibodies and their derivatives are used for clinical purposes in vitro and in vivo, especially for the diagnosis of cancer, for localization and in vivo imaging of tumors, for therapy, e.g. site-directed delivery of cytotoxins, and similar purposes. The invention also concerns test kits and pharmaceutical compositions containing said chimeric monoclonal antibodies and / or derivatives thereof.
Owner:CIBA GEIGY CORP
Immunogenic CEA
InactiveUS20050063952A1Effective immune responseBiocideGenetic material ingredientsVaccinationCarcinoembryonic antigen
The present invention provides for methods for immunizing actively against autologous carcinoembryonic antigen (CEA). The method encompasses that the immune system is engaged with variant CEA which is either administered as a protein vaccine, or is effected expressed by nucleic acid vaccination or live-viral vaccination. Preferred embodiments include immunization with variants that include at least one foreign T-helper epitope introduced in the CEA sequence. Also disclosed is variant proteins, DNA, vectors, and host cells useful for practising the method of the invention.
Owner:PHARMEXA
High affinity humanized anit-cea monoclonal antibodies
InactiveUS20020052479A1Reduce and potentially eliminate elicitingLow immunogenicityAnimal cellsIn-vivo radioactive preparationsCancer cellIn vivo
Novel humanized monoclonal antibodies, fragments or derivatives thereof which specifically bind carcinoembryonic antigen (CEA) are provided as well as methods for their manufacture. These humanized antibodies are useful in the treatment of cancers which express CEA as well as for diagnostic purposes, e.g., for in vivo imaging of tumors or cancer cells which express CEA.
Owner:THE DOW CHEM CO
Preparation and application of vaccine for curing tumor in positive carcino-embryonic antigen
ActiveCN1727362ALow costSimple stepsTumor rejection antigen precursorsPeptide/protein ingredientsCarcinoembryonic Antigen PositiveGenetic engineering
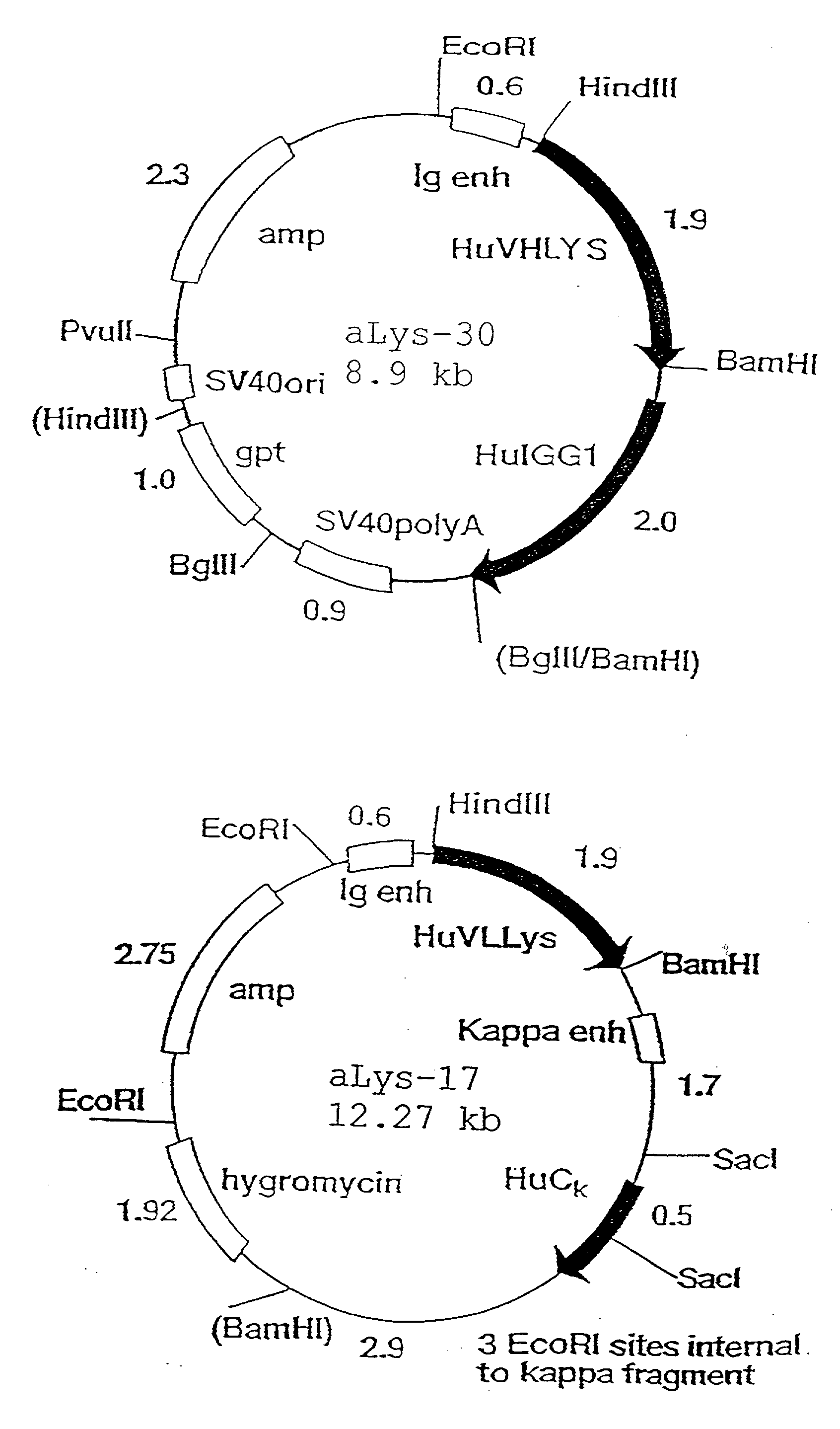
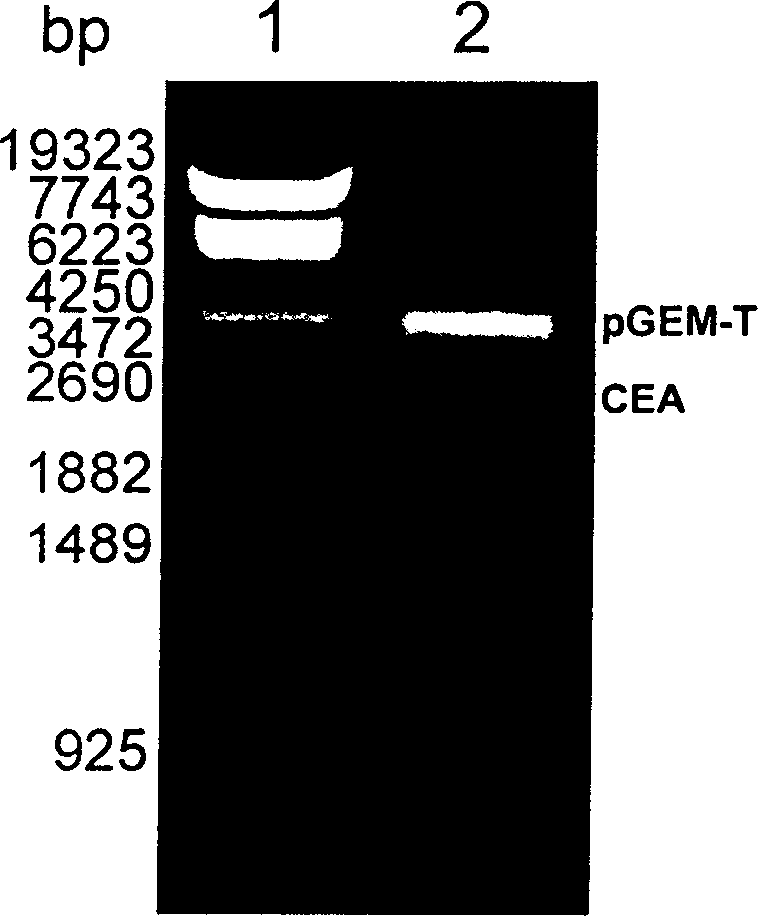
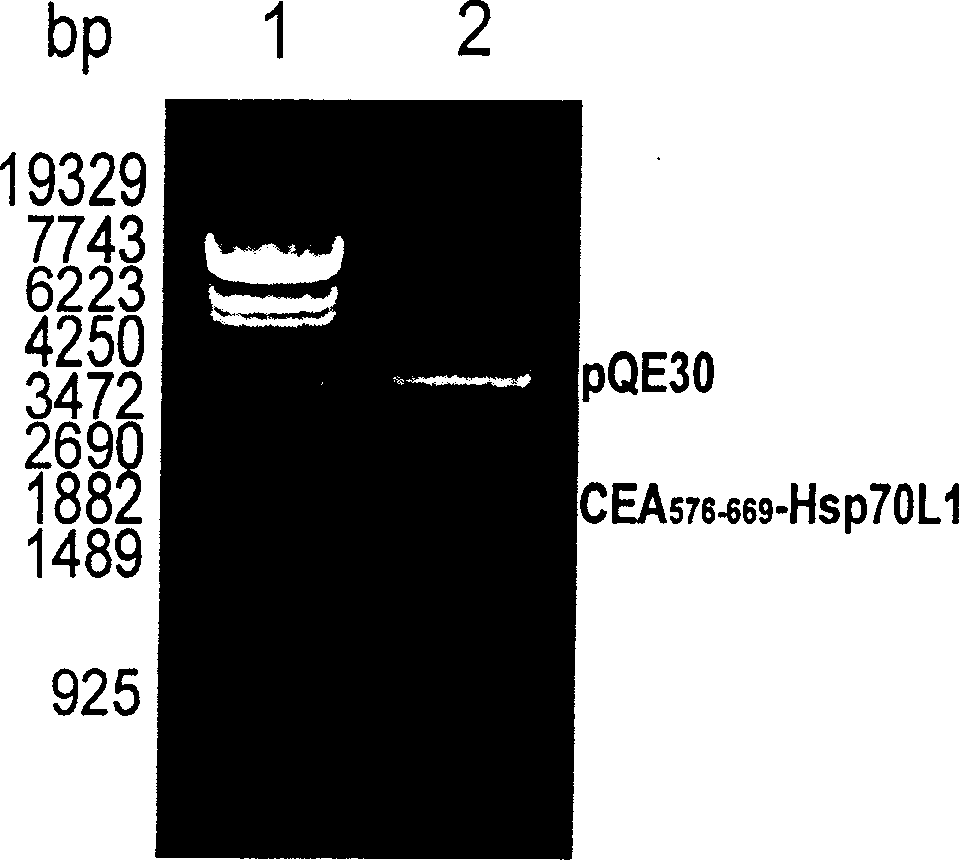
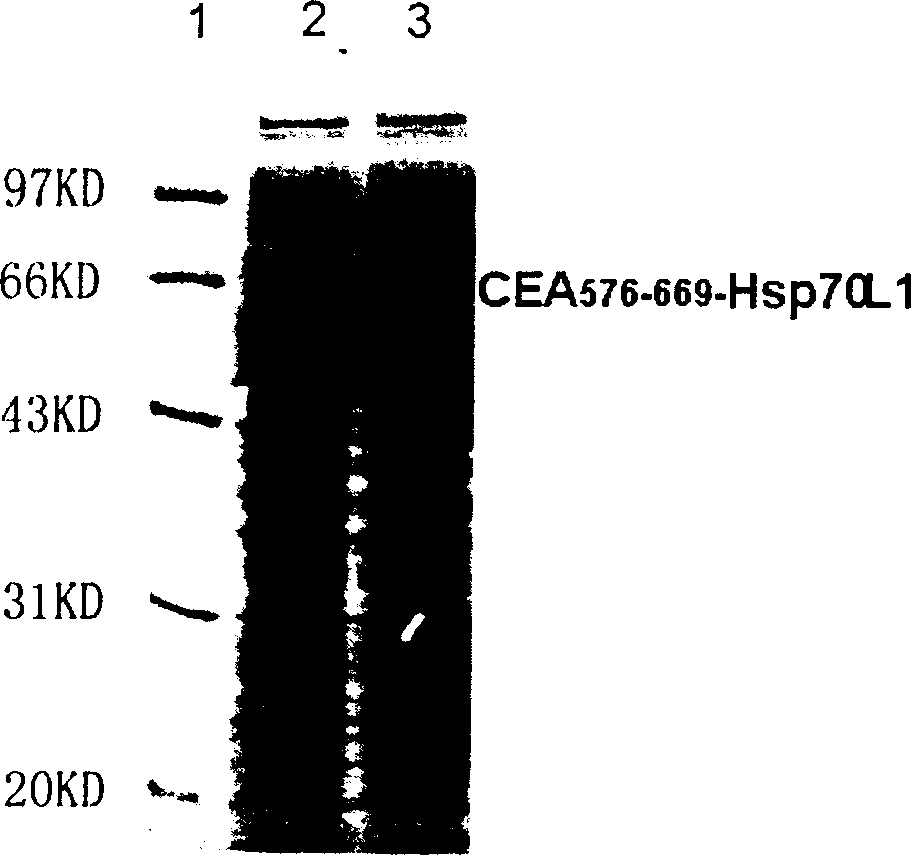
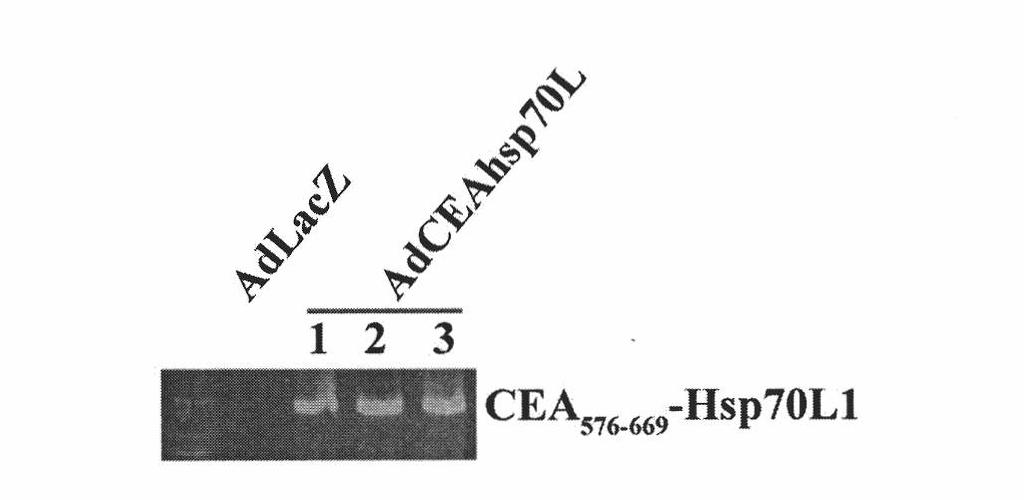
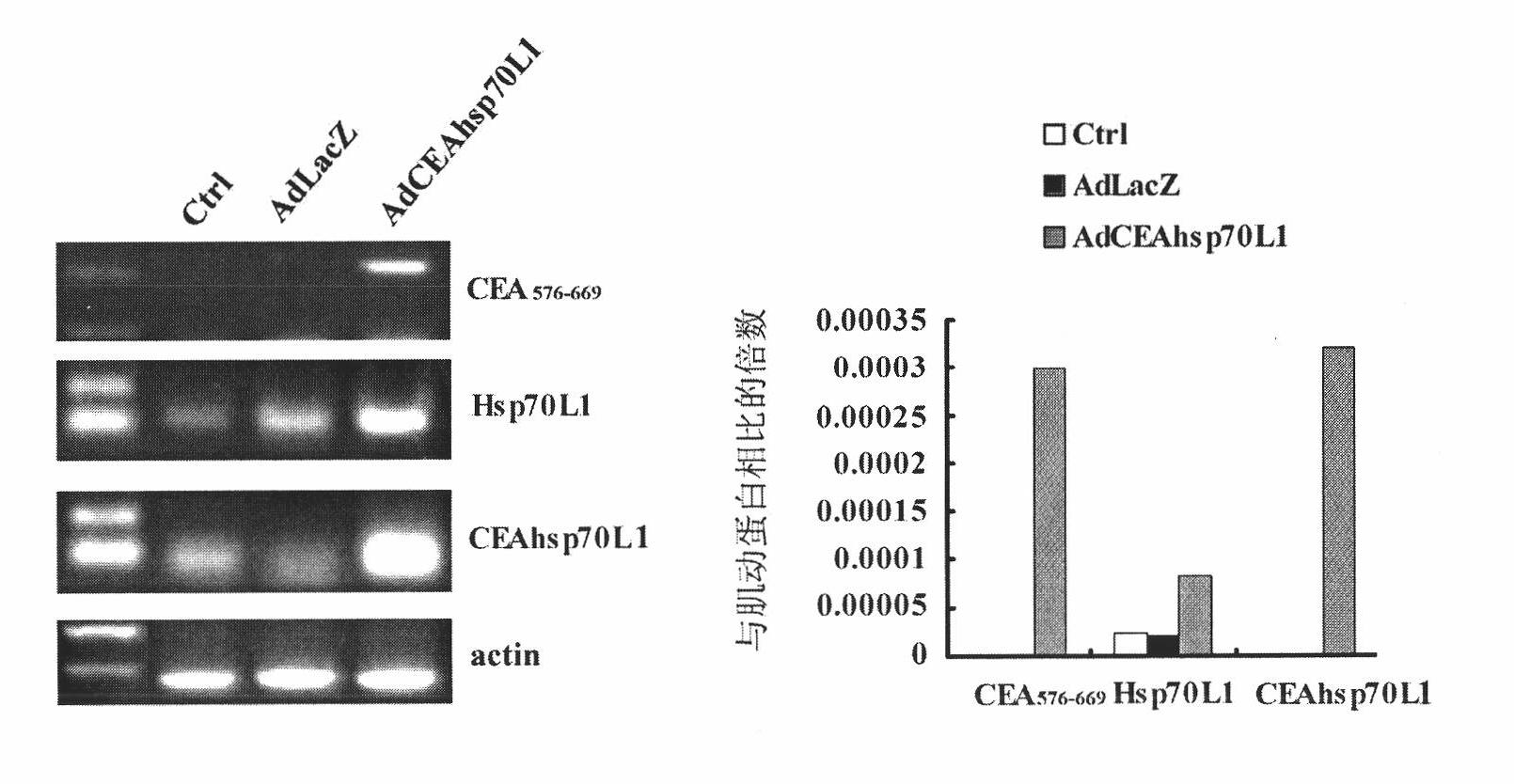
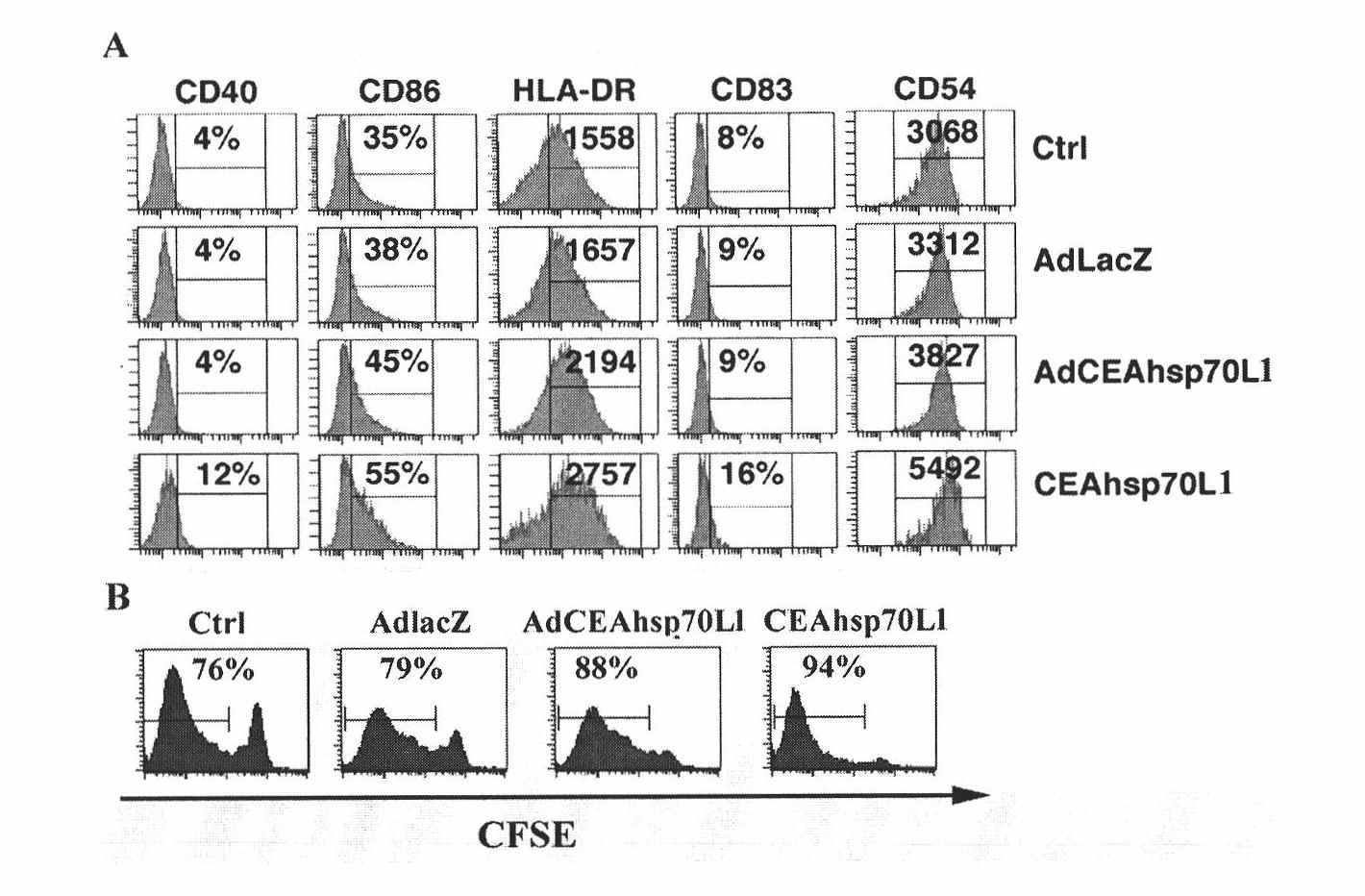
A fusion protein CEA-Hsp70L1 of human carcino-embryonic antigen and heat shock protein 70L1, the DNA sequence for coding it, the carrier containing the DNA sequence, the host cell containing said carrier, the process for preparing said fusion protein by genetic engineering, and the composition containing the fusion protein are disclosed. A process for preparing the therapeutic vaccine of CEA positive cancer is also disclosed.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
High-affinity anti-carcinoembryonic antigen nanobody and application thereof
ActiveCN108659130ASpecific recognition abilitySpecific bindingHydrolasesImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenCarcinoembryonic antigen
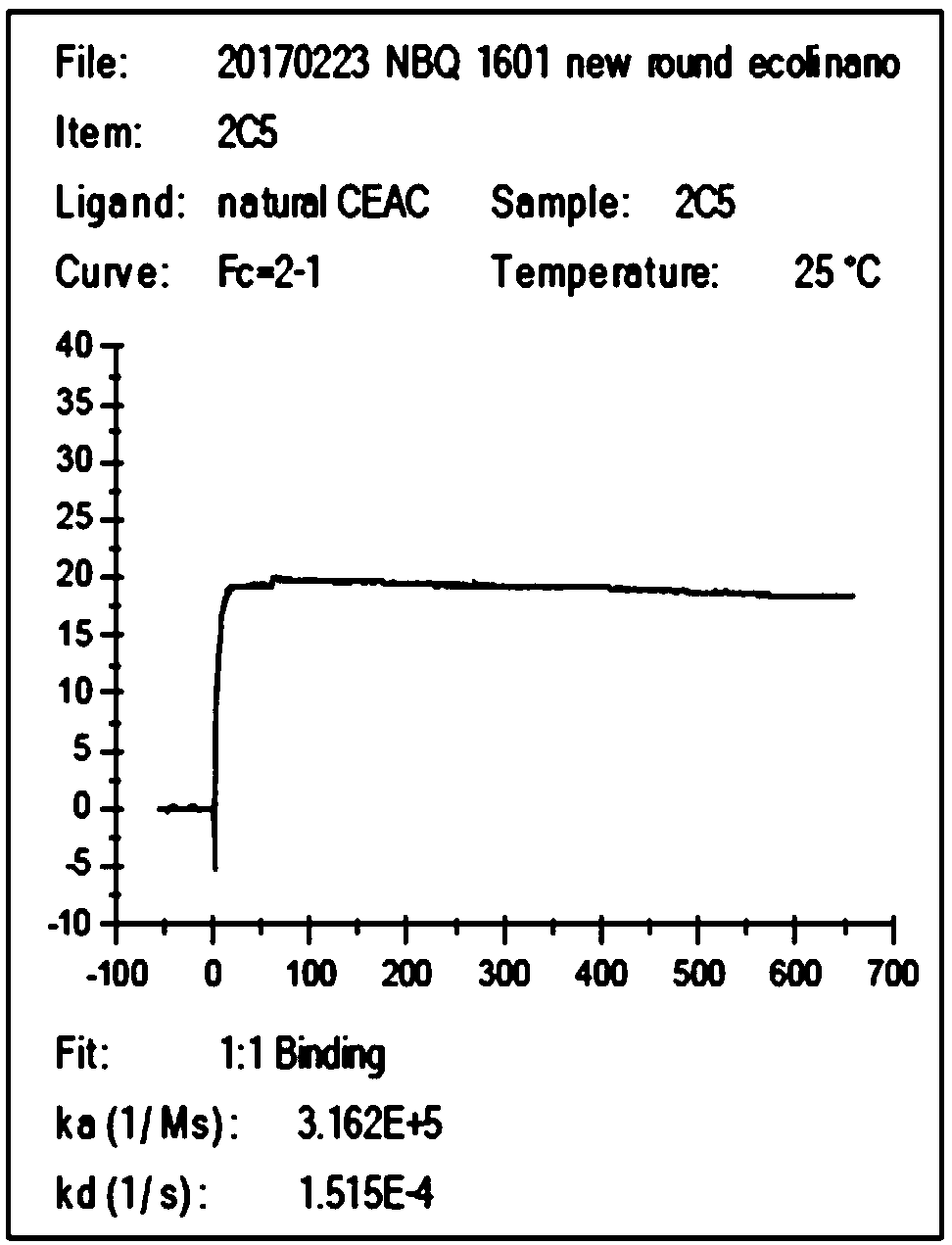
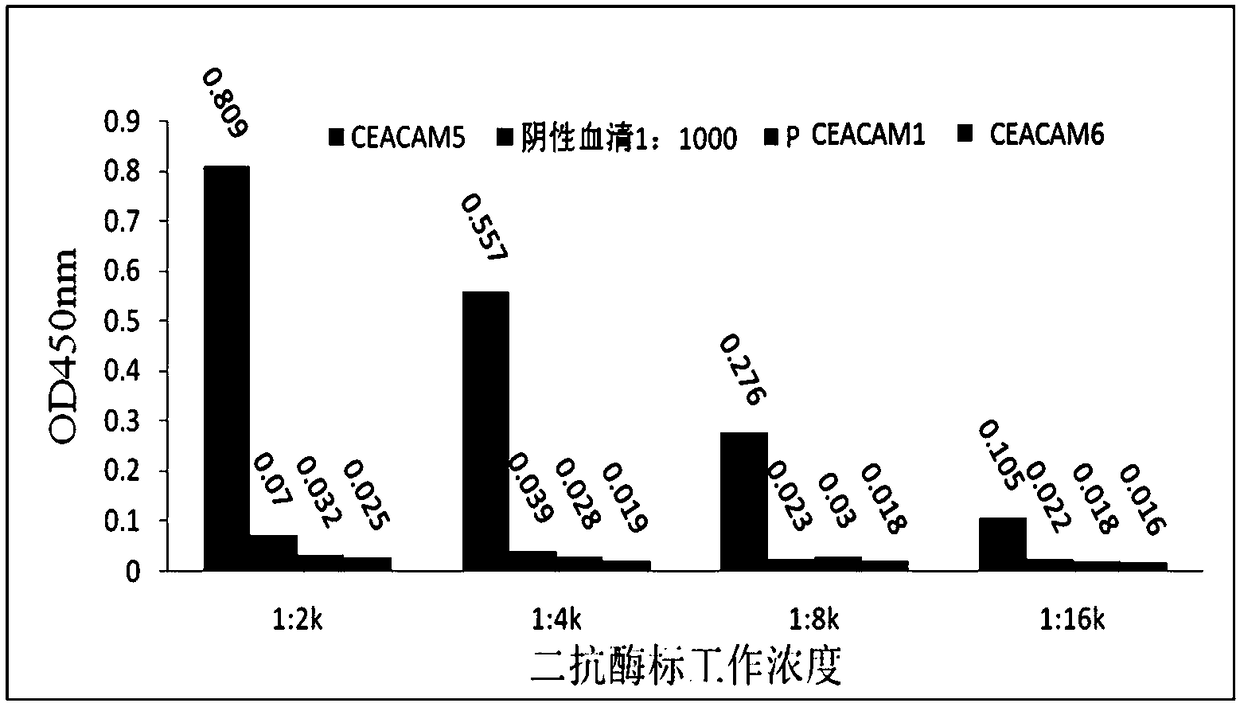
The invention discloses a high-affinity anti-carcinoembryonic antigen nanobody. The nanobody has three unique complementary determining regions CDR1, CDR2 and CDR3. The invention further provides an expression vector containing a nanobody variable region coding sequence and a host cell containing the expression vector, and further provides fusion protein of a nanobody variable region and alkalinephosphatase, the application of the nanobody to preparation of a carcinoembryonic antigen detection kit, a method for performing immunodetection on a carcinoembryonic antigen by applying the nanobody,and the corresponding detection kit. The anti-carcinoembryonic antigen (CEA) nanobody provided by the invention has a specific CEA recognizing and bonding ability, has the affinity which can reach 4.791E-10, and has unique antigen-determining cluster recognition sites; an excellent detection result can be obtained in CEA immunodetection, especially in a double-antibody sandwich method.
Owner:长春力太生物技术有限公司
Recombinant adenovirus with fusion protein gene and preparation method and application thereof
InactiveCN102154223ASubmission to achieveTumor rejection antigen precursorsAntibody mimetics/scaffoldsHsp70Recombinant adenoviruses
The invention relates to a recombinant adenovirus with a fusion protein gene and a preparation method and application thereof. Particularly, the invention relates to the recombinant adenovirus with coding sequences of a heat shock protein and a fusion protein of a carcinoembryonic antigen and the preparation method and the application thereof. An E1 region and an E3 region are deleted from a genome of the adenovirus and an expression box of the coding sequence of the fusion protein is inserted at the position of the E1 and / or E3 region. The expression box sequentially comprises a promotor sequence, the coding sequence of the fusion protein and a tailing signal sequence, wherein the heat shock protein is selected from Hsp70 or Hsp70L1. The recombinant adenovirus can be used for efficiently transferring and expressing a mediated gene (particularly tumor) and has wide application prospect.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Humanized Anti-cea t84.66 antibody and uses thereof
ActiveUS20080069816A1In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsCarcinoembryonic Antigen PositiveIntravenous gammaglobulin
Embodiments of the present invention utilize a more efficient CDR grafting technique to generate humanized versions of the T84.66 antibody. The technique used to generate these antibodies utilizes crystallographic structural data to select an immunoglobulin framework having maximum structural overlap with a non-human donor molecule. This technique was used to develop humanized T84.66 antibodies exhibiting in vitro binding affinity and specificity for carcinoembryonic antigen (CEA) nearly identical to that of T84.66 and the ability to specifically target tumors expressing CEA in vivo.
Owner:CITY OF HOPE
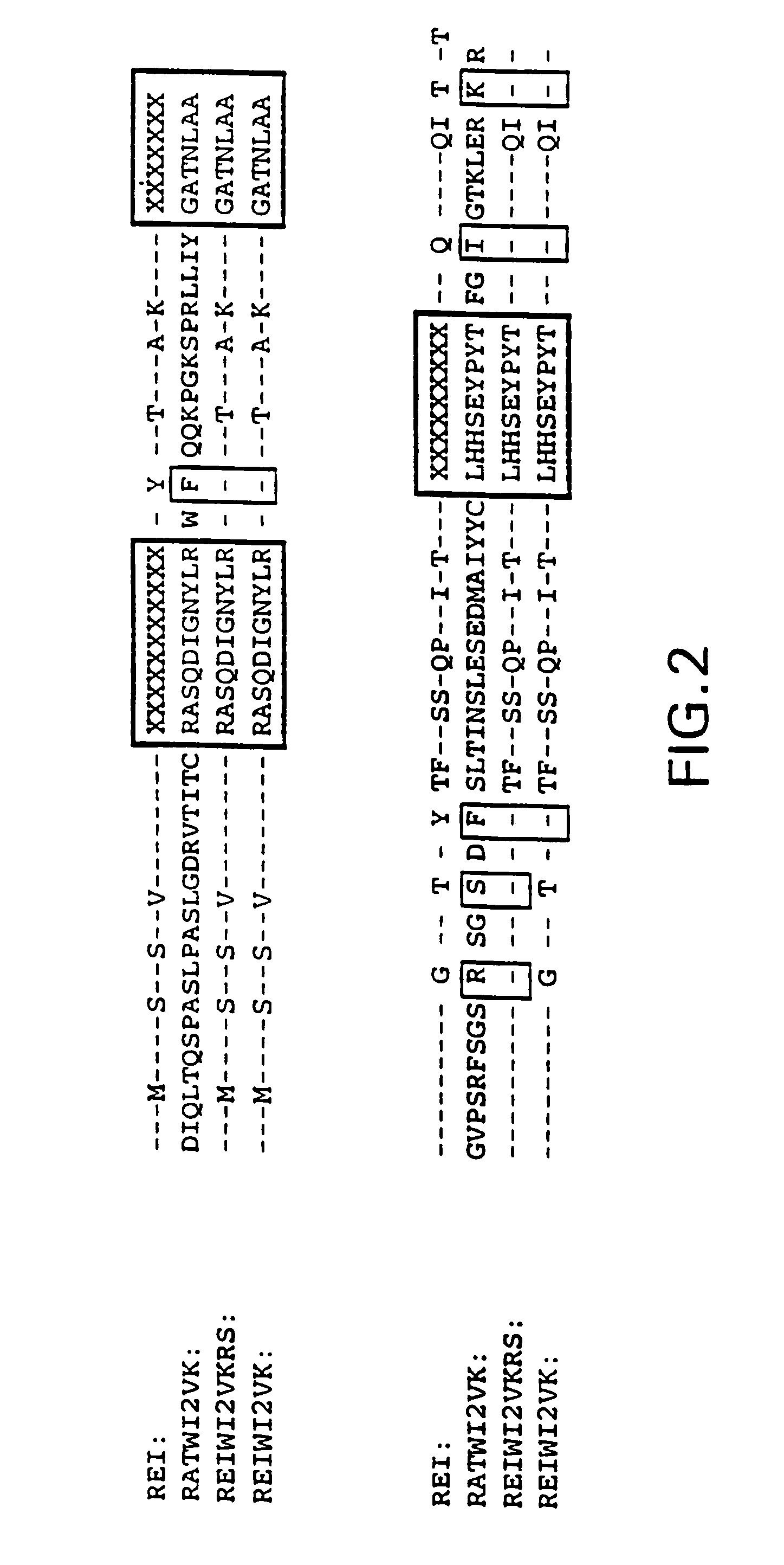
Murine monoclonal anti-idiotype antibody 3H1 sequences for human carcinoembryonic antigen
This invention provides compositions derived from the sequences encoding the variable light and / or variable heavy regions of monoclonal anti-idiotype antibody 3H1 and methods for using these compositions.
Owner:KENTUCKY UNIV OF THE BOARD OF TRUSTEES OF
Humanized anti-CEA T84.66 antibody and uses thereof
ActiveUS7273608B2In-vivo radioactive preparationsImmunoglobulins against cell receptors/antigens/surface-determinantsCarcinoembryonic Antigen PositiveIntravenous gammaglobulin
Embodiments of the present invention utilize a more efficient CDR grafting technique to generate humanized versions of the T84.66 antibody. The technique used to generate these antibodies utilizes crystallographic structural data to select an immunoglobulin framework having maximum structural overlap with a non-human donor molecule. This technique was used to develop humanized T84.66 antibodies exhibiting in vitro binding affinity and specificity for carcinoembryonic antigen (CEA) nearly identical to that of T84.66 and the ability to specifically target tumors expressing CEA in vivo.
Owner:CITY OF HOPE
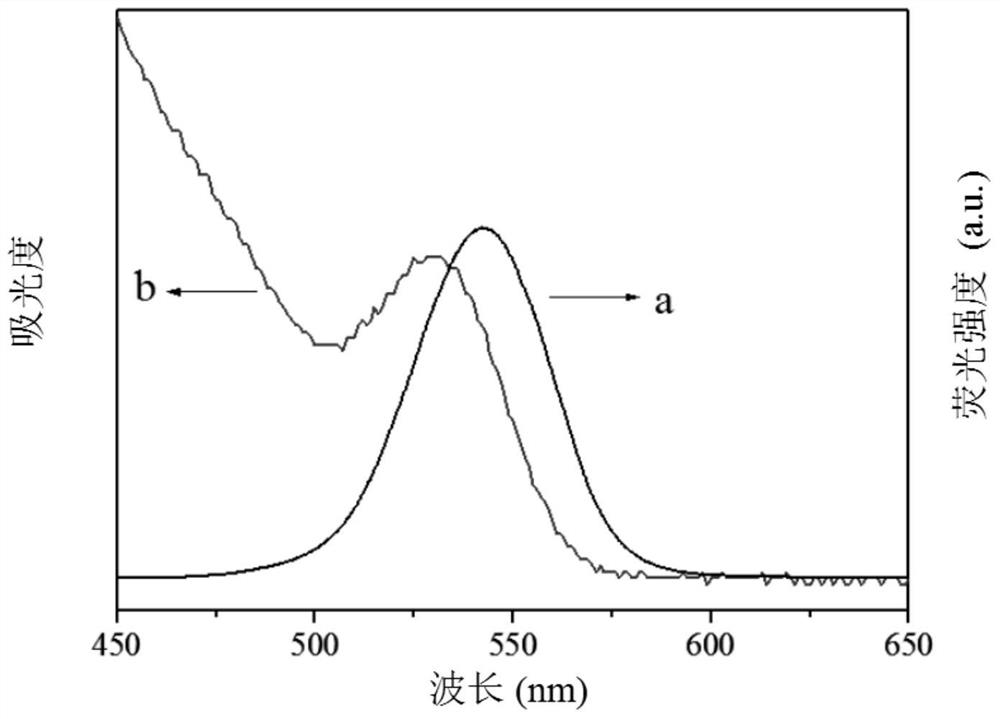
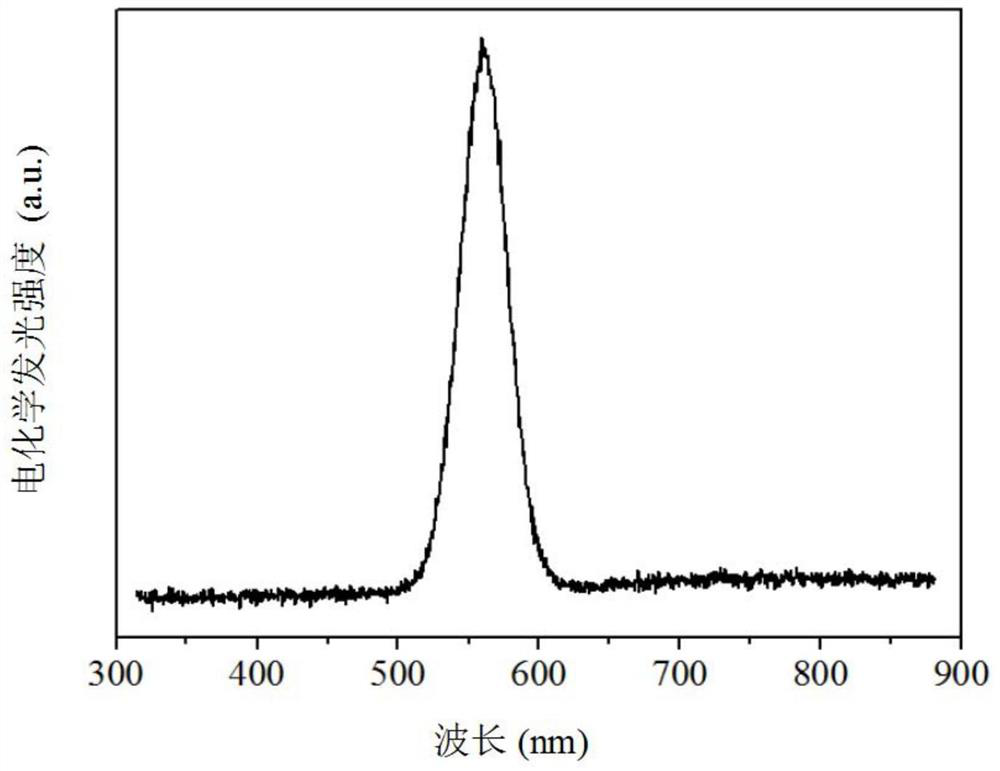
Electrochemiluminescence method for detecting carcinoembryonic antigen and kit thereof
InactiveCN109142331AHigh detection sensitivityAvoid interferenceChemiluminescene/bioluminescenceMaterial analysis by electric/magnetic meansQuantum yieldAntigen testing
The invention discloses an electrochemiluminescence method for detecting a carcinoembryonic antigen and a kit thereof. The method adopts the carcinoembryonic antigen as a detection object, organicallycombines a high quantum yield gold nanocluster electrochemiluminescence technology and an immunoassay technology, adopts a reduction method to prepare a high quantum yield gold nanocluster electrochemiluminescence probe, and uses a manganese dioxide nanomaterial as an electrochemiluminescence quencher to recover an electrochemiluminescence signal by an oxidation-reduction reaction of manganese dioxide and ascorbic acid produced by an enzyme-linked immunosorbent reaction, thereby developing the novel high-performance electrochemiluminescence carcinoembryonic antigen detection method based on high quantum yield gold nanocluster probes and the detection kit. The linear range of detection of a carcinoembryonic antigen in the method is 3*10-5 ng / mL-3 ng / mL, and the detection limit is 27 fg / mL.The method has the advantages of being fast, accurate, high in sensitivity, good in selectivity and stability, low in sample consumption and the like, and has a good clinical application prospect.
Owner:FUJIAN MEDICAL UNIV
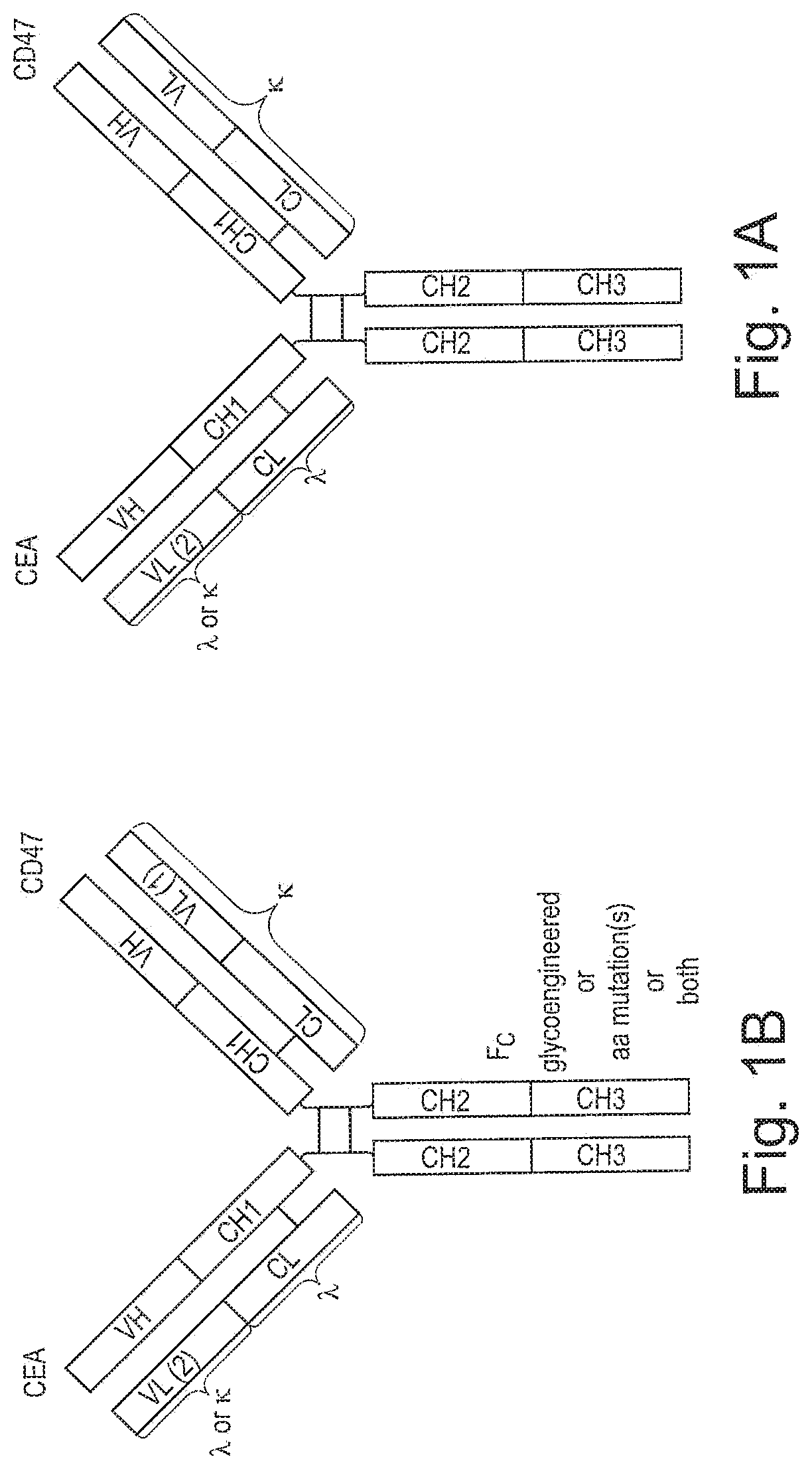
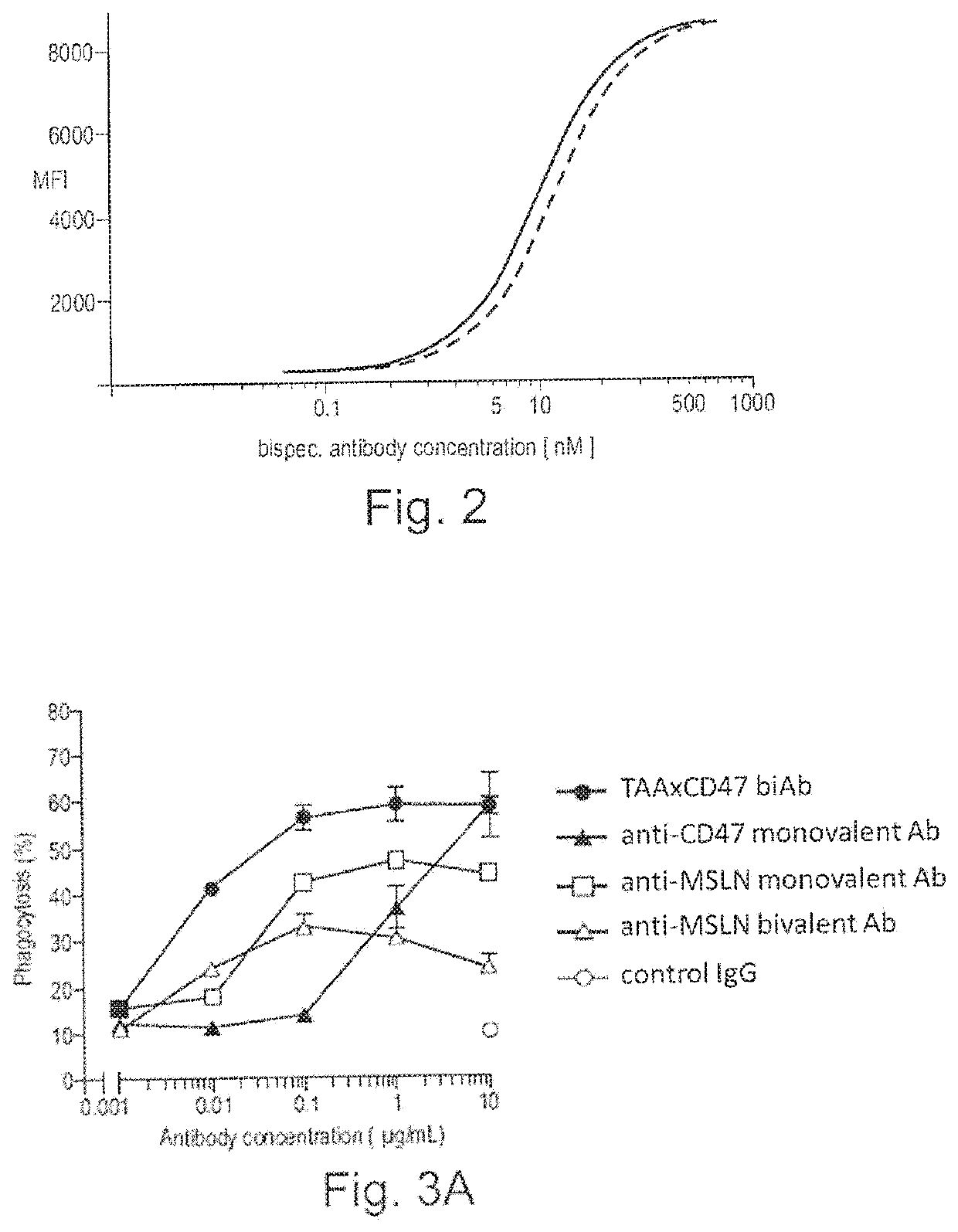
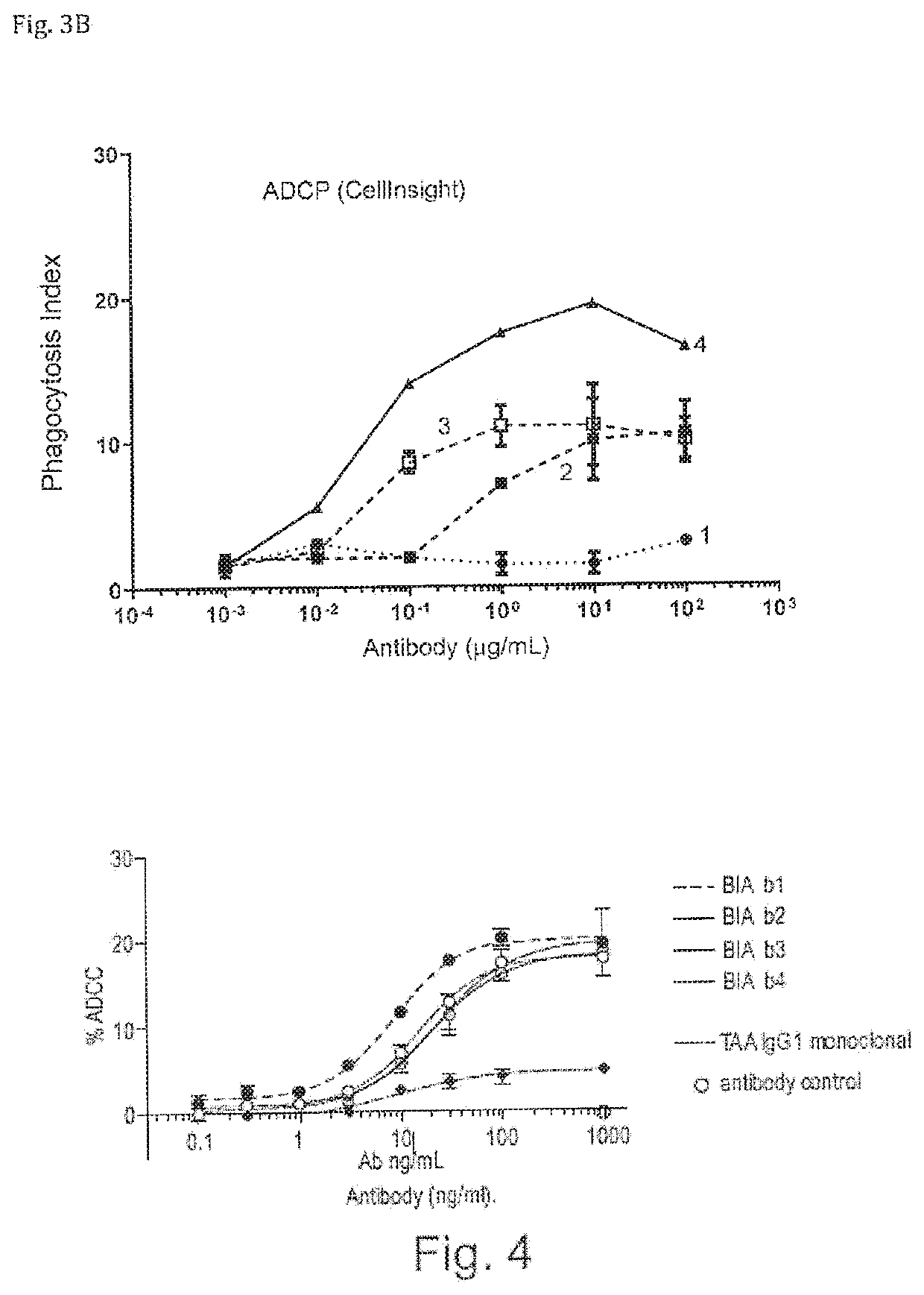
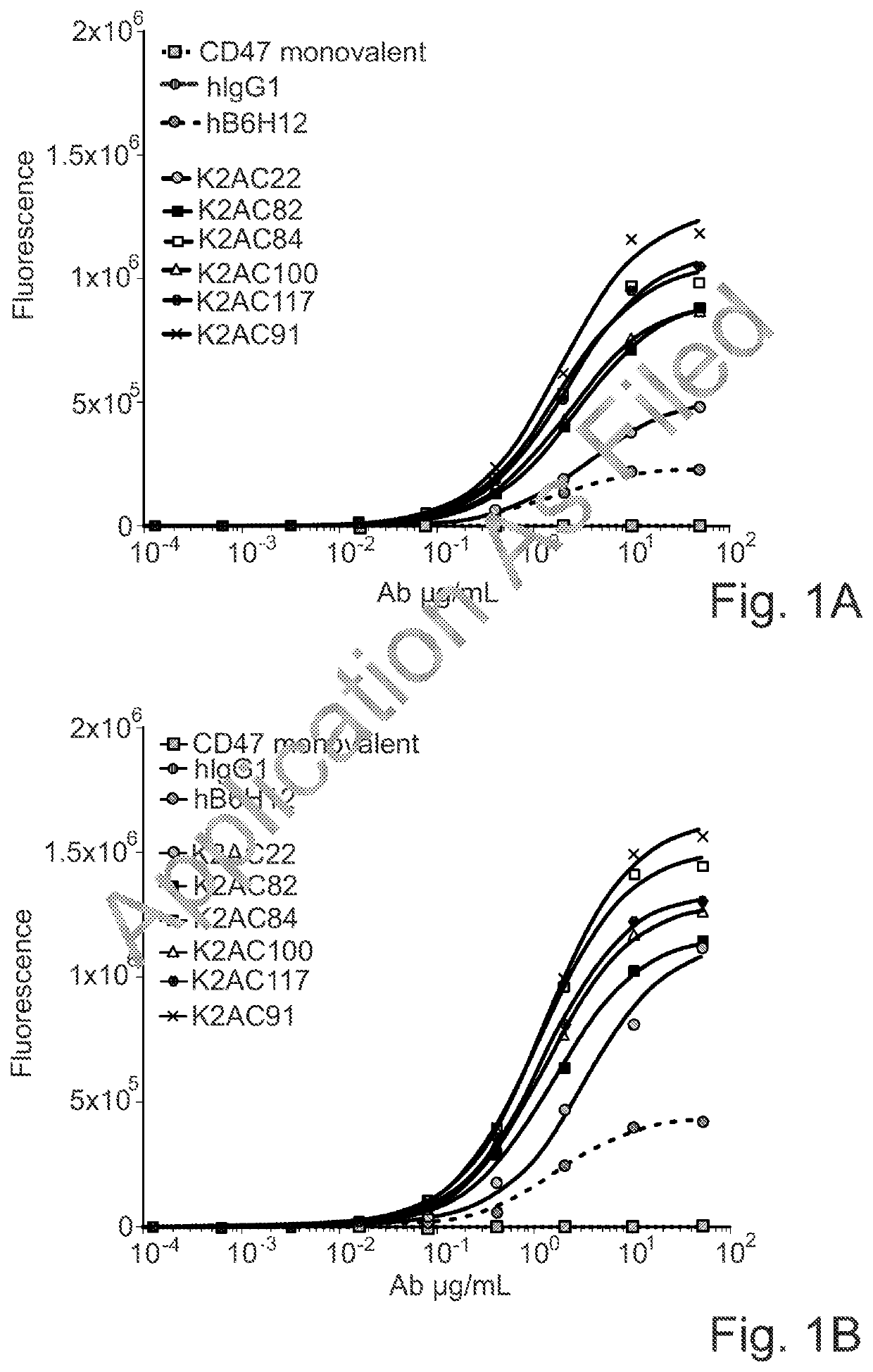
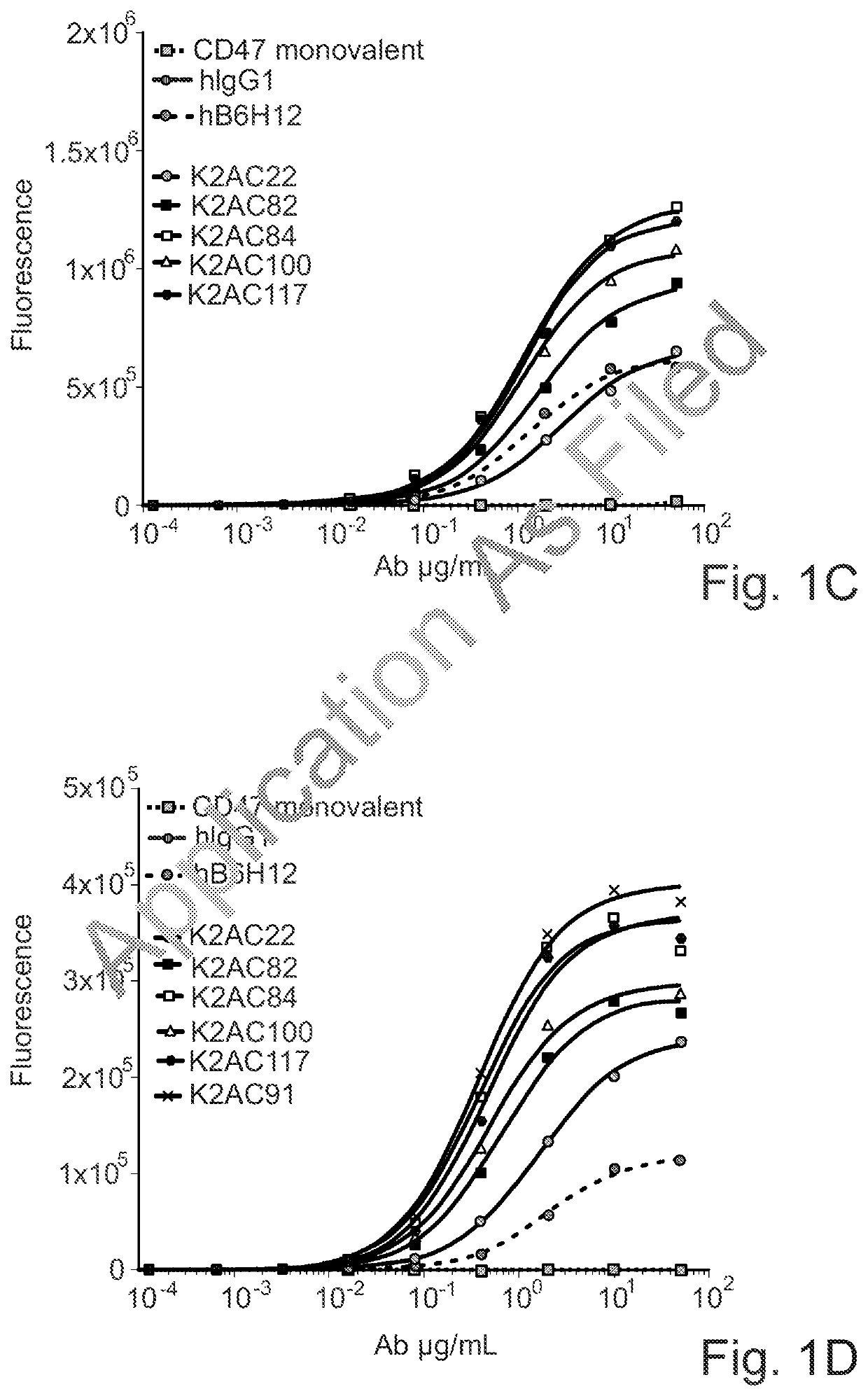
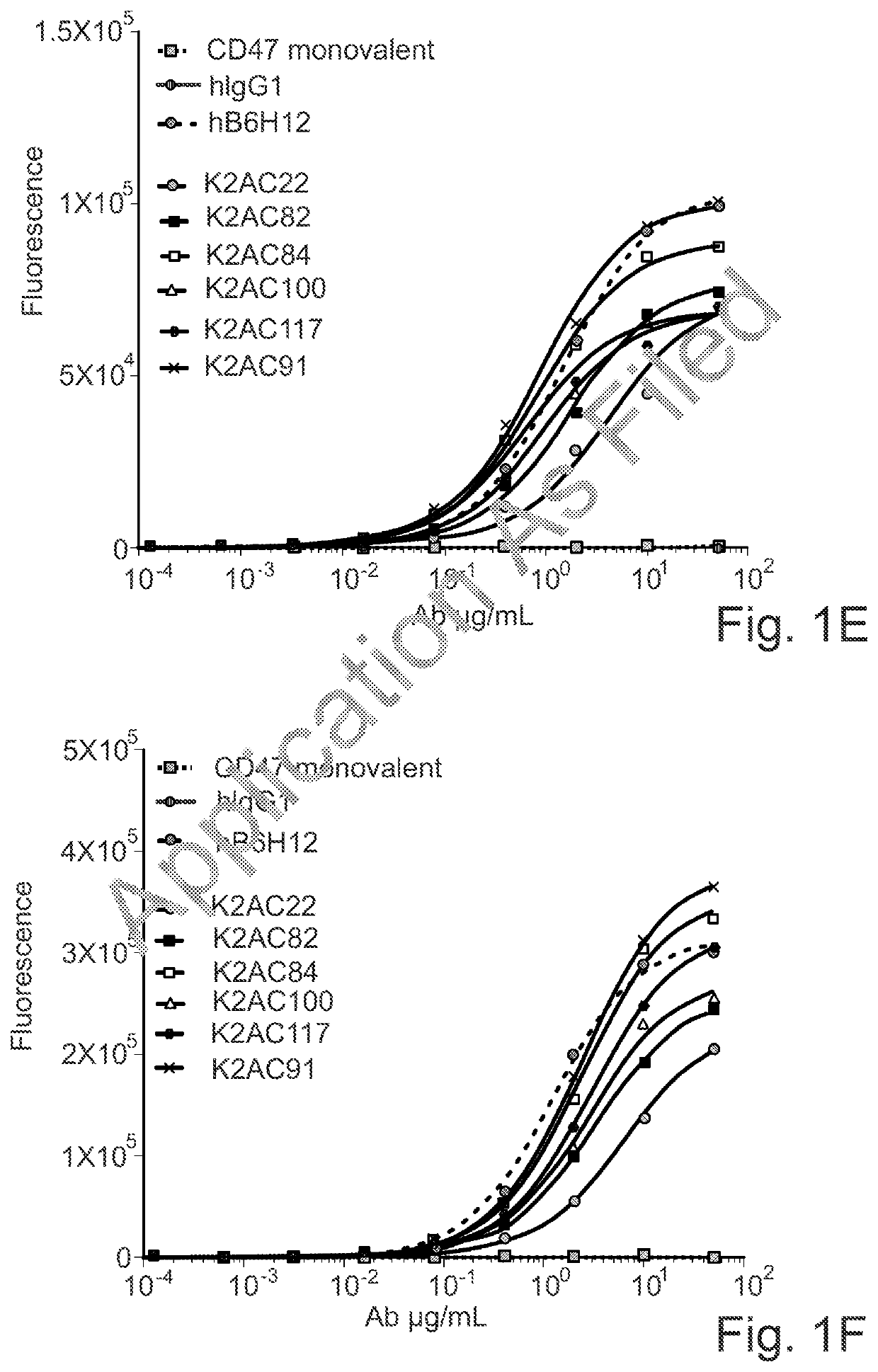
Bispecific antibodies against ceacam5 and cd47
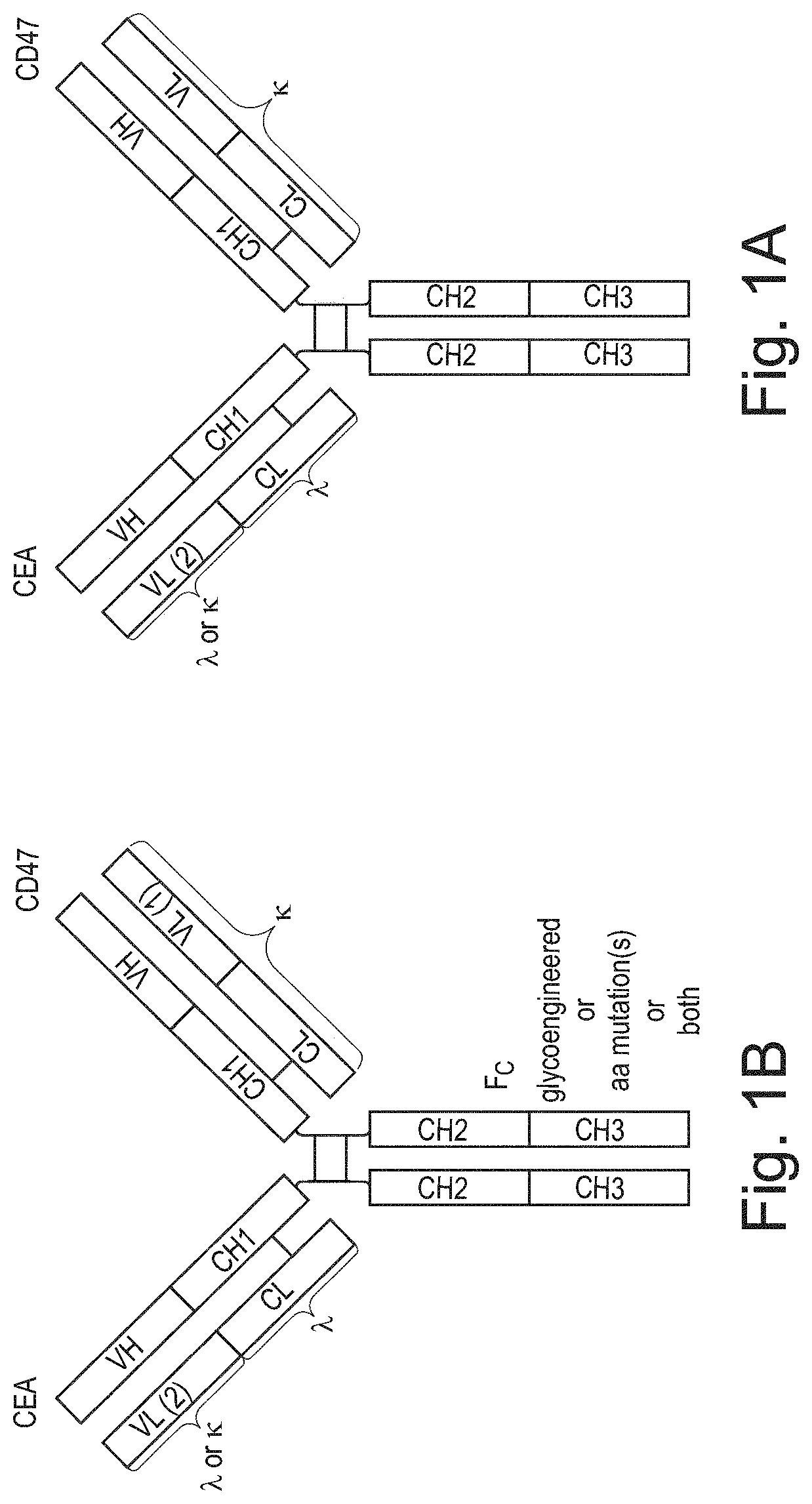
ActiveUS20200123252A1Impairment of activityFunction increaseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseCarcinoembryonic Antigen Positive
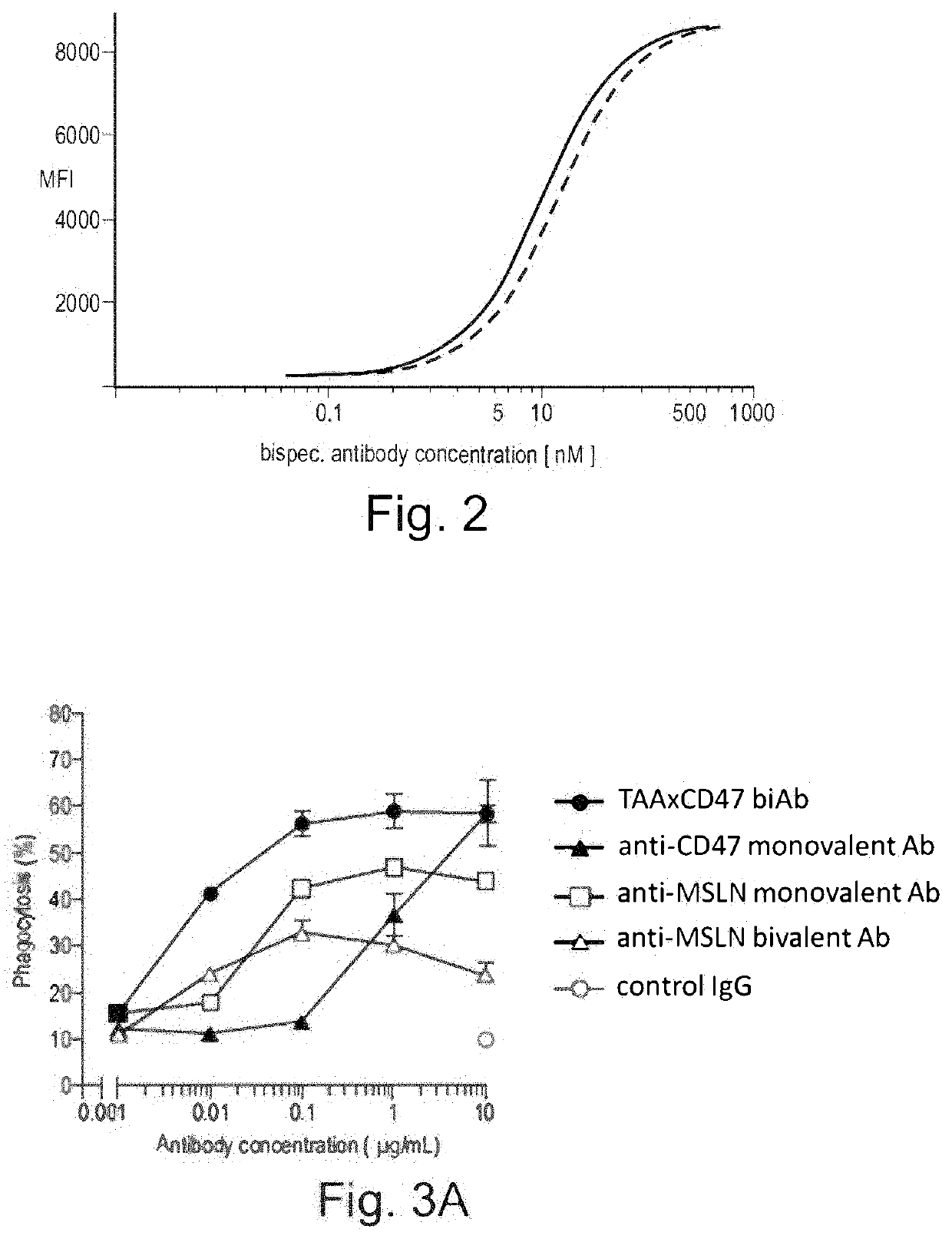
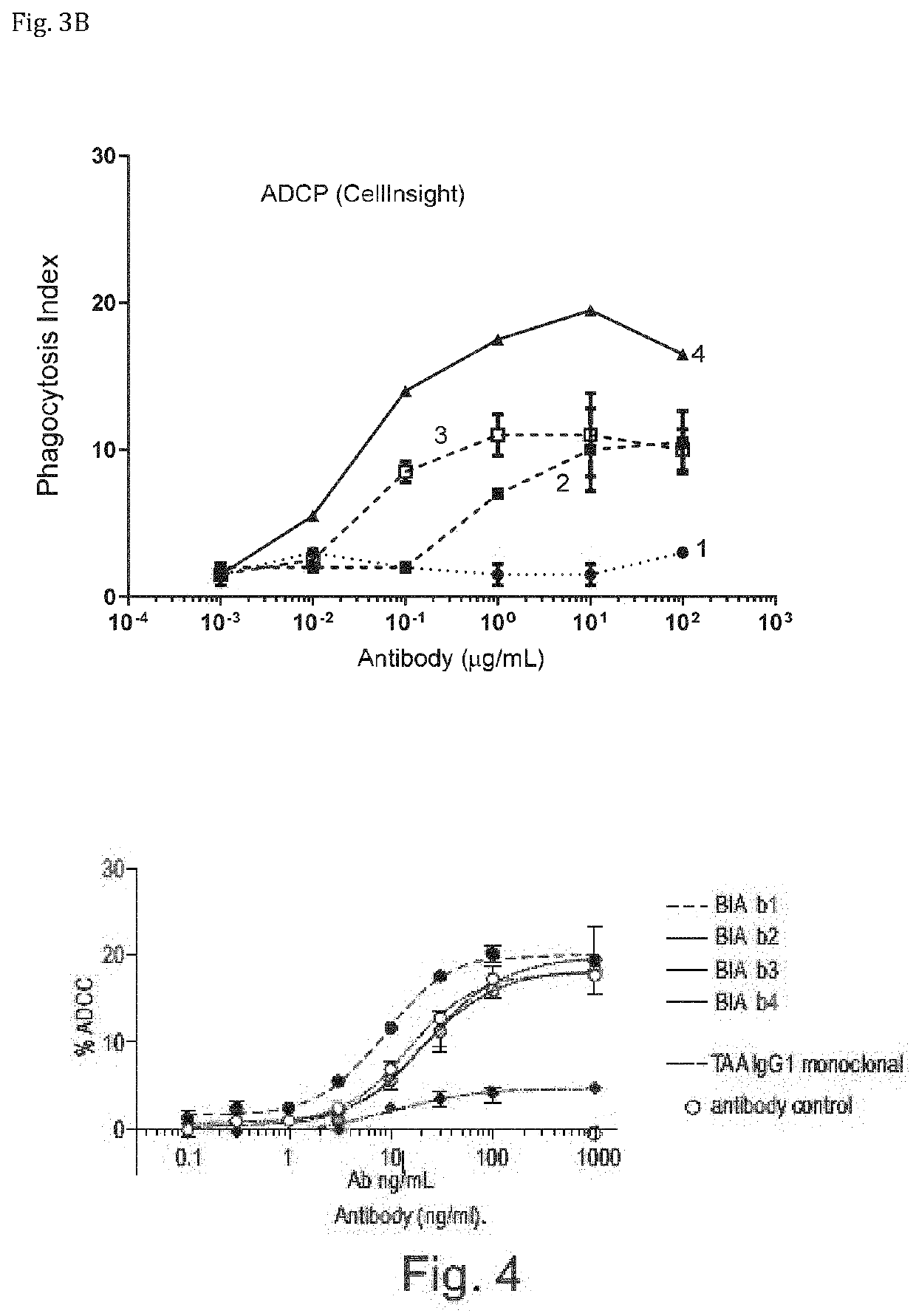
The invention provides bispecific antibodies binding to human carcinoembryonic antigen CEACAM5 and human CD47, polynucleotides encoding such bispecific antibodies and vectors and host cells comprising such polynucleotides. The invention further provides methods for selecting and producing such antibodies and methods of using such antibodies in the treatment of diseases in monotherapy as well in combination.
Owner:LAMKAP BIO BETA LTD
Recombinant immunotoxin based on pseudomonas exotoxin containing (Arg) 9 peptide segment
InactiveCN1676531AImprove internalization efficiencyEnhance cell killing activityAntibody ingredientsHybrid peptidesAntigenPseudomonas aeruginosa exotoxin A
This invention belongs to anti-carcinoe mbryonic antigen recombination immuno toxin. It is concretely relates to said antigen immuno toxic with (Arg)9 based on pseudomonic extotoxin. Nucleuotide sequence code-connects ectotoxin and the antigen connecting peptide, contains the expressing carrier of the sequence and the xeno-cell of the sequence and the utilization of the prevention and cure of tumor.
Owner:BEIJING ABT GENETIC ENG TECH
Carcinoembryonic antigen (CEA) rapid simple detection kit and making and application methods thereof
The aim of the invention is to provide a carcinoembryonic antigen (CEA) rapid simple detection kit and making and application methods thereof, the carcino-embryonic antigen rapid simple detection kit is lower in cost than a test strip; by use of specific responses of human carcinoembryonic antigen and anti carcinoembryonic antigen antibody, the carcinoembryonic antigen and the antibody are bonded onto a NC (nitrocellulose) membrane and colloidal gold particles, a colloidal gold and antibody complex is added onto the NC membrane, and whether the CEA is negative or positive is determined by observation of the color. When the CEA content is lower than 2.5uM, a 2.5uM CEA standard product is not significantly different from a 0uM standard product in color development, so when the CEA content is lower than 2.5uM, the CEA is determined to be negative; when the CEA content is in the 2.5-10uM range, the detection point color is significantly shallower than the 0uM spot color, the CEA is determined to be weak positive; when the CEA content is more than 10uM, and no red spot appears, the CEA is determined to be positive.
Owner:UNIV OF ELECTRONICS SCI & TECH OF CHINA
Antibody resisting carcinoembryonic antigen and preparation method and application of antibody
ActiveCN110655581AIncrease lethalityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsComplementarity determining regionSingle-Chain Antibodies
The invention provides a CEA resisting antibody. The CEA resisting antibody comprises a heavy chain variable region and a light chain variable region, wherein the complementary determining region of the heavy chain variable region comprises CDR-H1 of which the amino acid sequence is shown as SEQID No.1, CDR-H2 of which the amino acid sequence is shown as SEQID No.2 and CDR-H3 of which the amino acid sequence is shown as SEQID No.3, and the complementary determining region of the light chain variable region comprises CDR-L1 of which the amino acid sequence is shown as SEQID No.4, CDR-L2 of which the amino acid sequence is shown as SEQID No.5 and CDR-L3 of which the amino acid sequence is shown as SEQID No.6. The inventor uses a phage display technique to screen CEA scFv, so as to obtain a high-affinity CEA resisting single-chain antibody. The chimeric antigen receptor gene for resisting CEA is transmitted into T cells by a genetic engineering method to prepare CEA CAR-T, so that the digestive tract tumor cells of CEA can be specially recognized and expressed by CEA CAR-T cells, and are killed, and the effect of resisting tumors can be realized.
Owner:HUADAO SHANGHAI BIOPHARMA CO LTD
Carcinoembryonic antigen positive cell targeted gene expression element CPD and application thereof
InactiveCN101638654APrevent proliferationGenetic material ingredientsAntineoplastic agentsAbnormal tissue growthCarcinoembryonic Antigen Positive
The invention belongs to biotechnologies and relates to regulation and control of gene expression, in particular to design and preparation of a targeted gene expression element CPD specifically aimingat a carcinoembryonic antigen positive tumor cell and specific expression in the carcinoembryonic antigen positive tumor cell. A product can effectively block DNA replication in the tumor cell so that a cell cycle can not be carried out due to the blockage of the DNA replication, and the effect of inhibiting cell proliferation can be achieved. The invention can be used for preparing a targeted tumor gene treating medicine. The invention has the advantages of favorable tumor inhibiting effect, wide adaptability and high safety.
Owner:杭州安倍生物科技有限公司
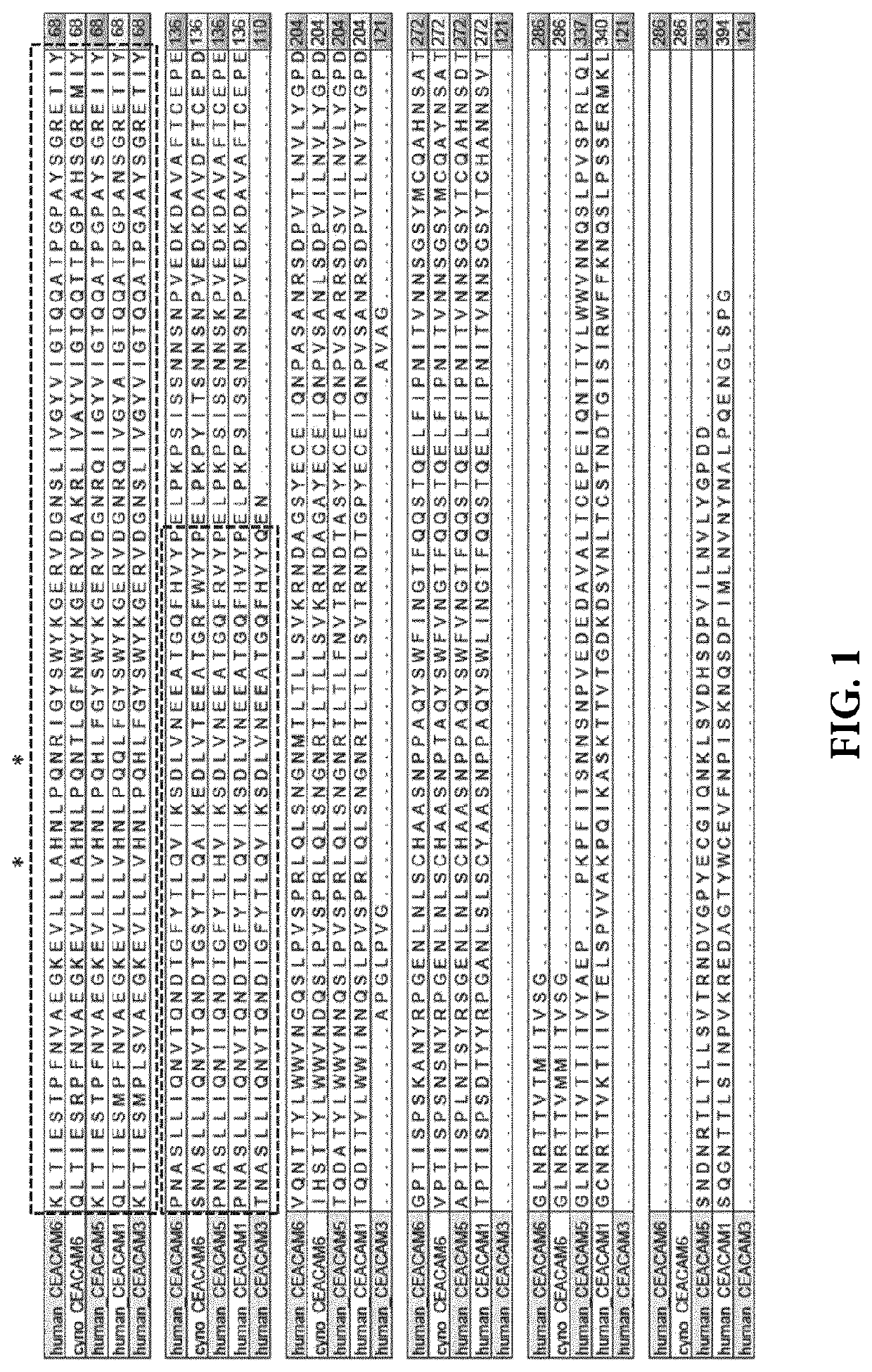
Anti-CEACAM6 antibodies and uses thereof
ActiveUS10584167B2Relieve CEACAM6-mediated immunosuppressionIncreased IFN-gammaImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAntiendomysial antibodies
The present invention provides recombinant antigen-binding regions and antibodies and functional fragments containing such antigen-binding regions that are specific for human and Macaca fascicularis CEACAM6 (Carcinoembryonic antigen-related cell adhesion molecule 6, CD66c, Non-specific crossreacting antigen, NCA, NCA-50 / 90), and which do not significantly cross-react with the closely related human CEACAM1, human CEACAM3, and human CEACAM5. The invention further provides methods to generate this kind of antibodies. The antibodies, accordingly, can be used to treat cancer and other disorders and conditions associated with expression of the CEACAM6. The invention also provides nucleic acid sequences encoding the foregoing antibodies, vectors containing the same, pharmaceutical compositions and kits with instructions for use.
Owner:BAYER PHARMA AG
Bispecific antibodies against ceacam5 and cd47
PendingUS20220195067A1Reduce rateRaise the ratioImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesCarcinoembryonic Antigen Positive
The present invention relates to bispecific antibodies which bind to human carcinoembryonic antigen CEACAM5 and human CD47. In addition, the present invention relates to polynucleotides encoding such bispecific antibodies and vectors and host cells comprising such polynucleotides. The invention further relates to methods for selecting and producing such antibodies and to methods of using such antibodies in the treatment of diseases. The invention also relates to the therapeutic use of the bispecific antibodies in monotherapy and in combination therapy.
Owner:LAMKAP BIO BETA LTD
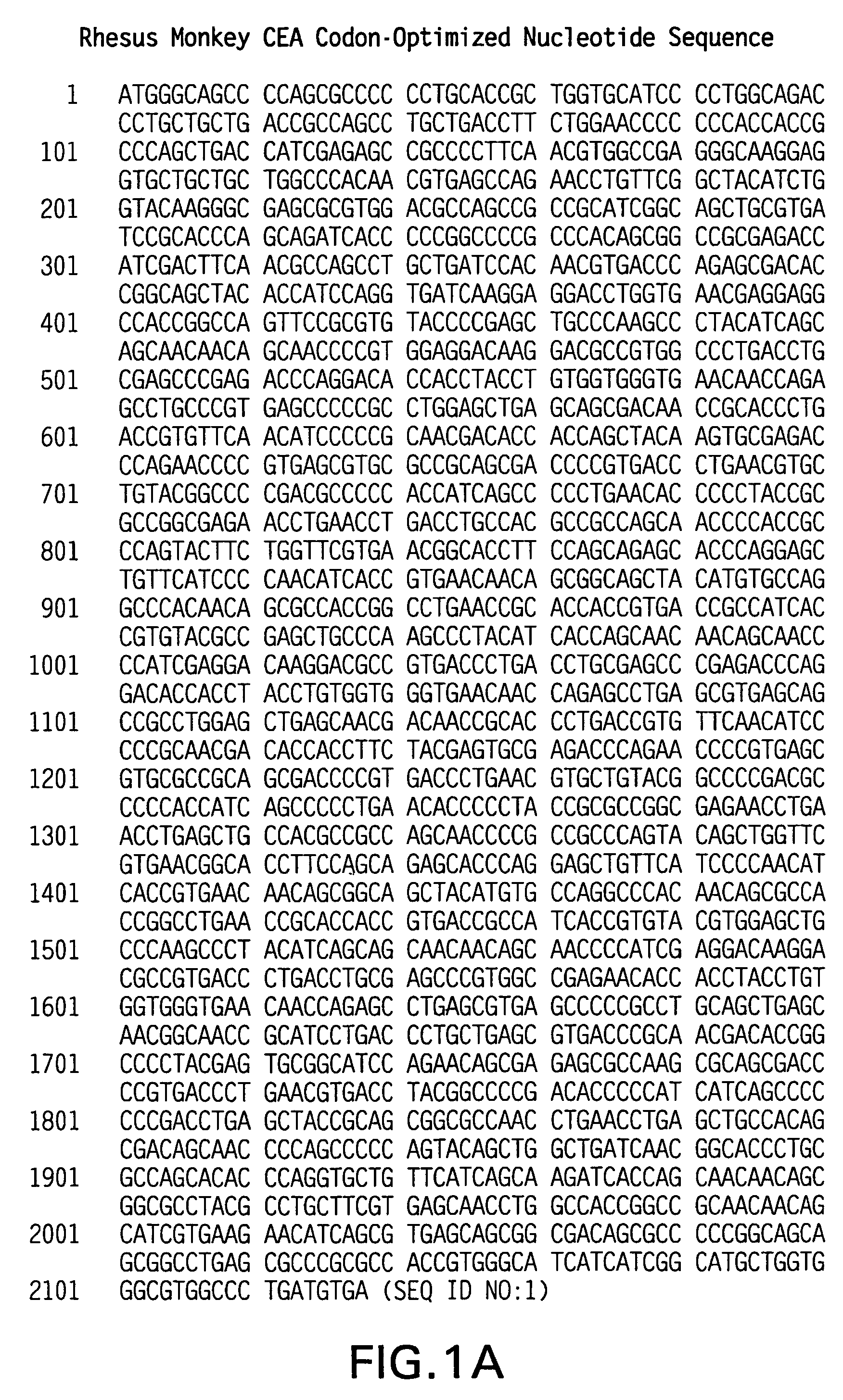
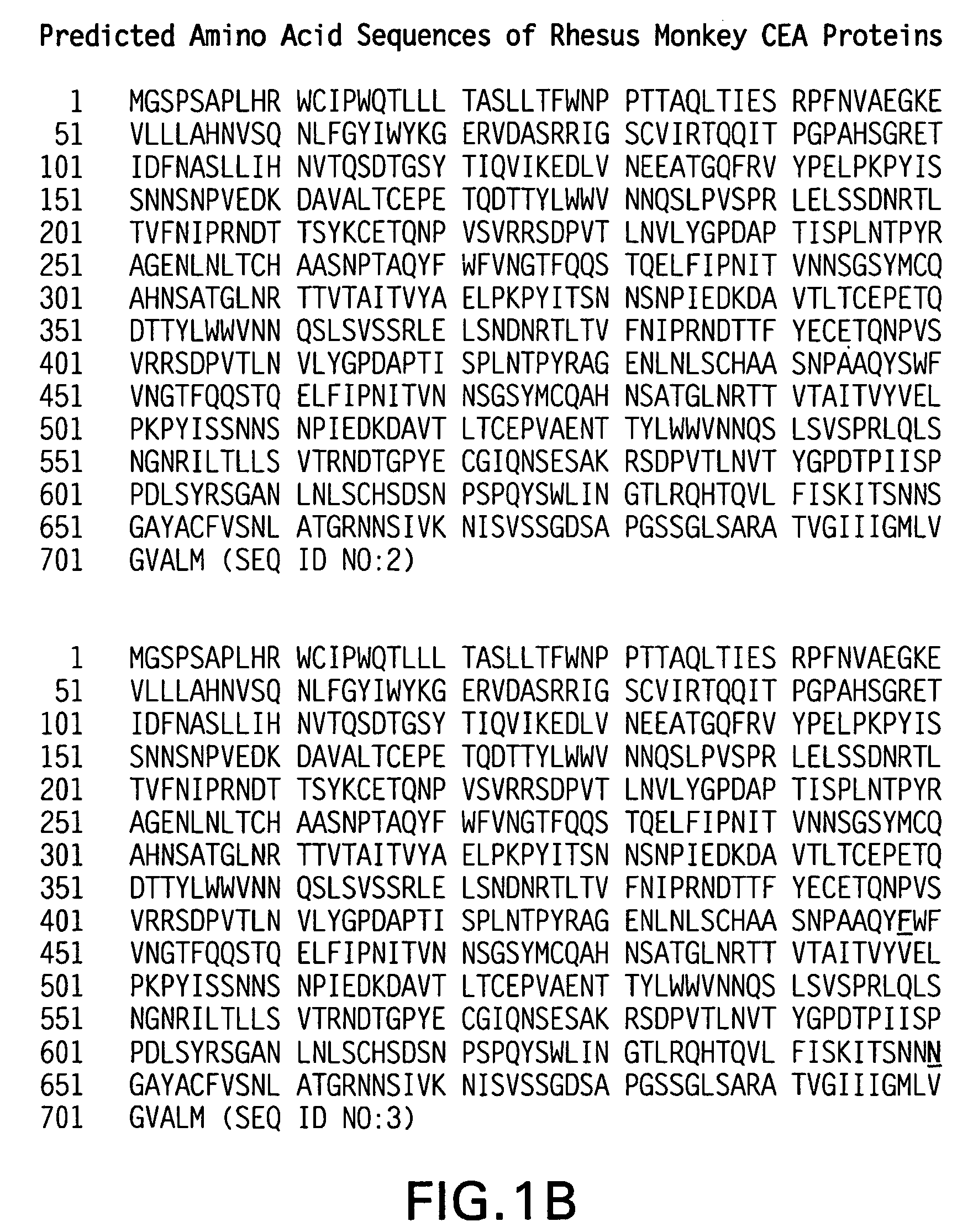
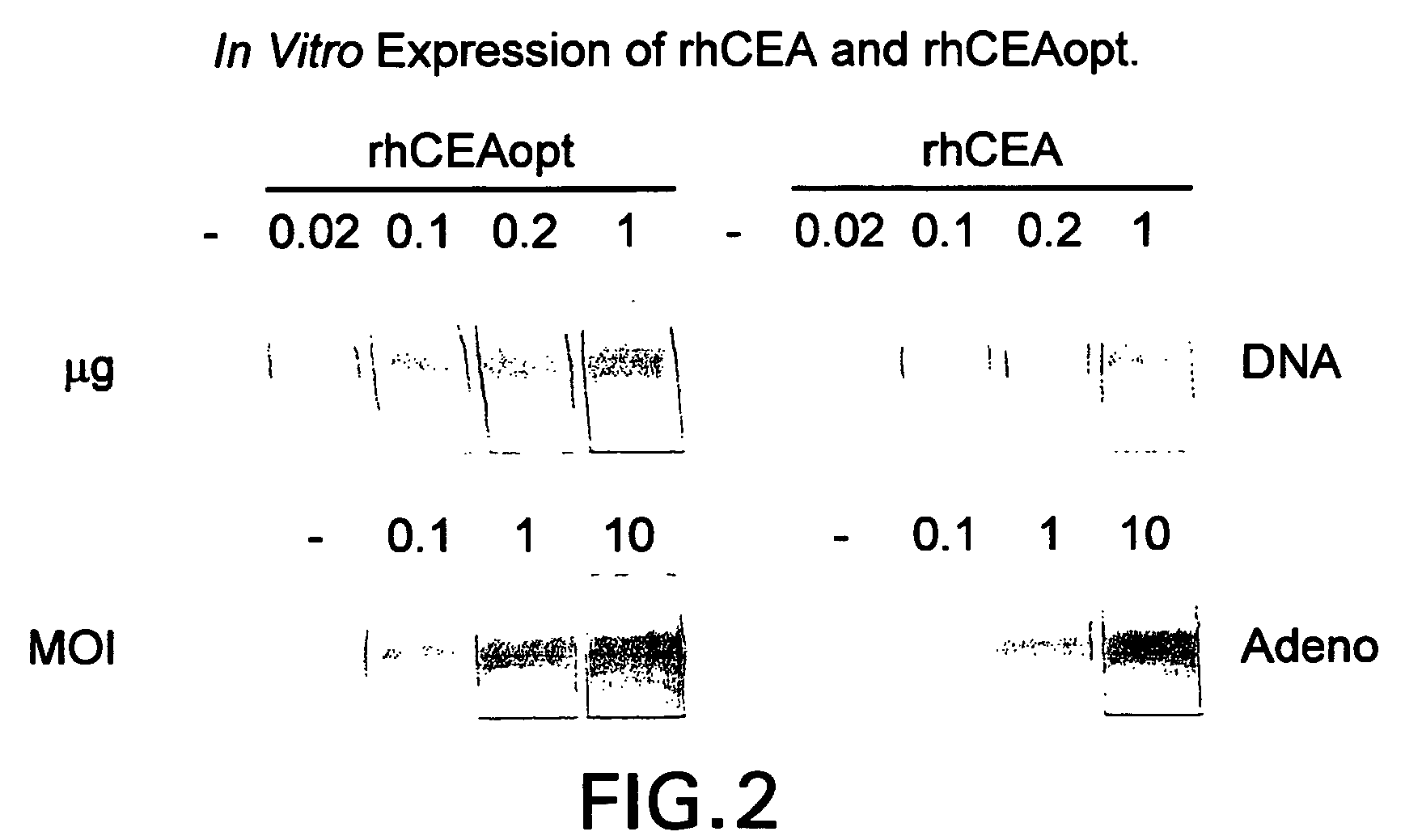
Synthetic gene encoding rhesus monkey carcinoembryonic antigen and uses thereof
InactiveUS20060286114A1Easy to insertEasy to removeTumor rejection antigen precursorsGenetic material ingredientsCarcinoembryonic antigenEukaryotic plasmids
Synthetic polynucleotides encoding rhesus monkey carcinoembryonic antigen (CEA) are provided, the synthetic polynucleotides being condon-optimized for expression in a human cellular environment. The gene encoding CEA is commonly associated with the development of human carcinomas. The present invention provides compositions and methods to elicit or enhance immunity to the protein product expressed by the CEA tumor-associated antigen, wherein aberrant CEA expression is associated with a carcinoma or its development. This invention specifically provides adenovial vector and plasmid constructs carrying codon-optimed rhesus monkey CEA and discloses their use in vaccines and pharmaceutical compositions for preventing and treating cancer.
Owner:IST DI RICERCHE DI BIOLOGIA MOLECOLARE P ANGELETTI
Carcinoembryonic antigen positive cell targeted gene expression element CPE and application thereof
InactiveCN101638655APrevent proliferationInhibitory cycle blockGenetic material ingredientsAntineoplastic agentsTumor targetAbnormal tissue growth
The invention relates to a novel tumor targeted gene expression element, named CPE. The element as a DNA molecule can be specifically expressed in a carcinoembryonic antigen positive tumor cell and can effectively block DNA replication in the tumor cell so that a cell cycle can not be carried out due to DNA replication blockage, and the effect of specifically inhibiting tumor cell proliferation can be achieved. The invention can be used for preparing a tumor gene treating medicine.
Owner:杭州安倍生物科技有限公司
Tumor gene diagnosis marker combined with carcinoembryonic antigen and application of tumor gene diagnosis marker
PendingCN113817824AHigh screening accuracyMicrobiological testing/measurementMaterial analysisCarcinoembryonic Antigen PositiveOncology
Owner:NANJING FANYIDA BIOTECHNOLOGY CO LTD
Bispecific antibodies against ceacam5 and cd47
InactiveUS20210221908A1Function increaseHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseCarcinoembryonic Antigen Positive
The invention provides bispecific antibodies binding to human carcinoembryonic antigen CEACAM5 and human CD47, polynucleotides encoding such bispecific antibodies and vectors and host cells comprising such polynucleotides. The invention further provides methods for selecting and producing such antibodies and methods of using such antibodies in the treatment of diseases inmonotherapy as well in combination.
Owner:LAMKAP BIO BETA LTD
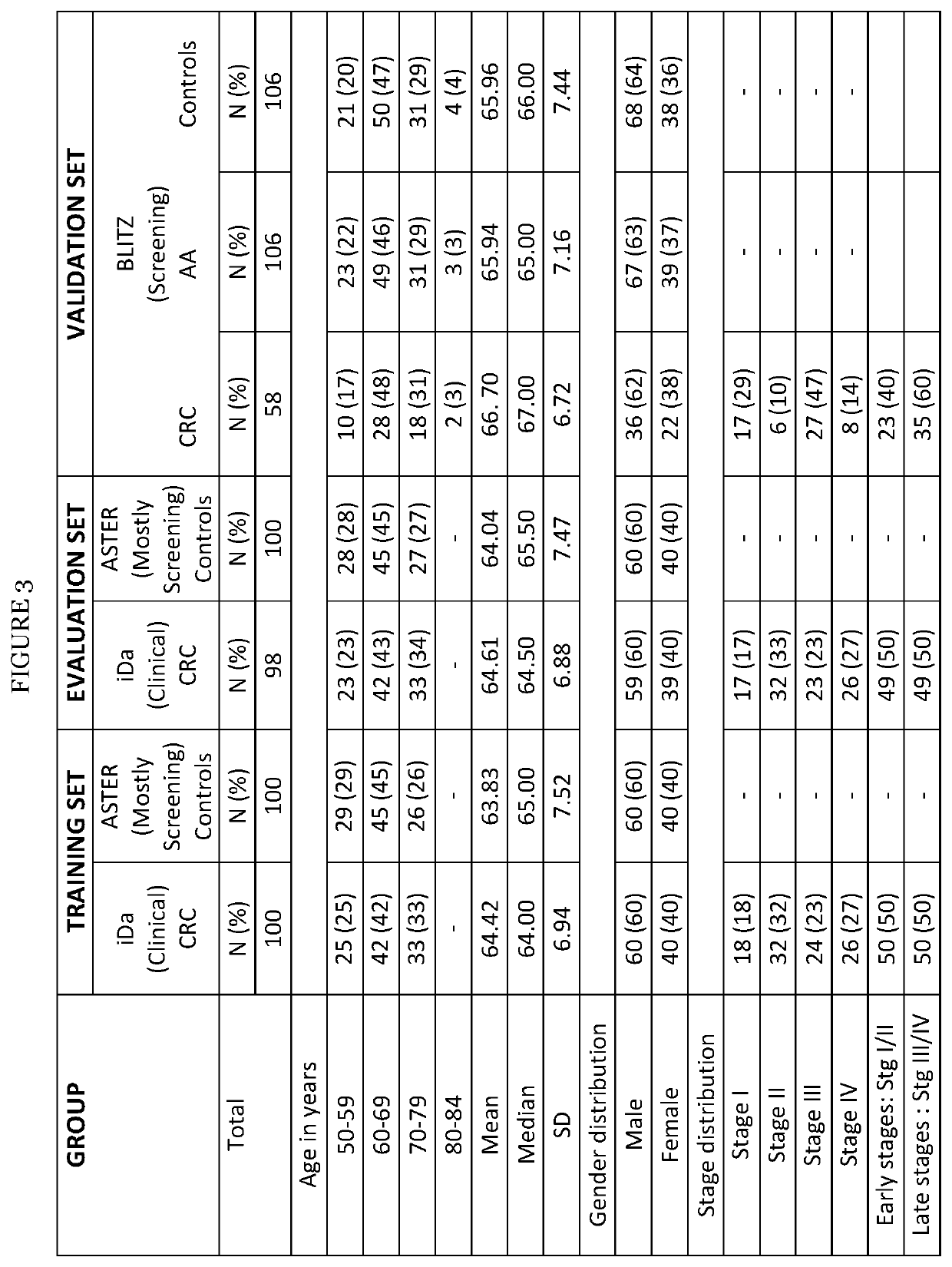
Colorectal cancer screening examination and early detection method
PendingUS20220214345A1Contributes immensely to the global burden of cancersReduce incidenceDisease diagnosisDiseaseBiomarker panel
The present invention pertains to a new method for the diagnosis, prognosis, stratification and / or monitoring of a therapy, of cancer, preferably colorectal cancer (CRC), in a subject. The method is based on the determination of the level of a panel of least one, preferably 3, 4 and most preferably at least 5, protein biomarker selected from the group consisting of the protein biomarkers Amphiregulin (AREG), Carcinoembryonic antigen (CEA), Insulin like growth factor binding protein 2 (IGFBP2), Keratin, type I cytoskeletal 19 (KRT19), Mannan binding lectin serine protease 1 (MASP1), Osteopontin (OPN), Serum paraoxonase lactonase 3 (PON3) and Transferrin receptor protein 1 (TR), in the biological sample obtained from the subject. The new biomarker panel of the invention allows diagnosing and even stratifying various cancer diseases. Furthermore, provided are diagnostic kits for performing the non-invasive methods of the invention. Since the biomarker panel of the invention provides a statistically robust method independent of the protein detection technology used, and considering that the biomarker panel of the invention is detected in plasma samples of the subjects, the invention provides an early detection screening examination that may be applied to a larger population.
Owner:DEUTES KREBSFORSCHUNGSZENT STIFTUNG DES OFFENTLICHEN RECHTS
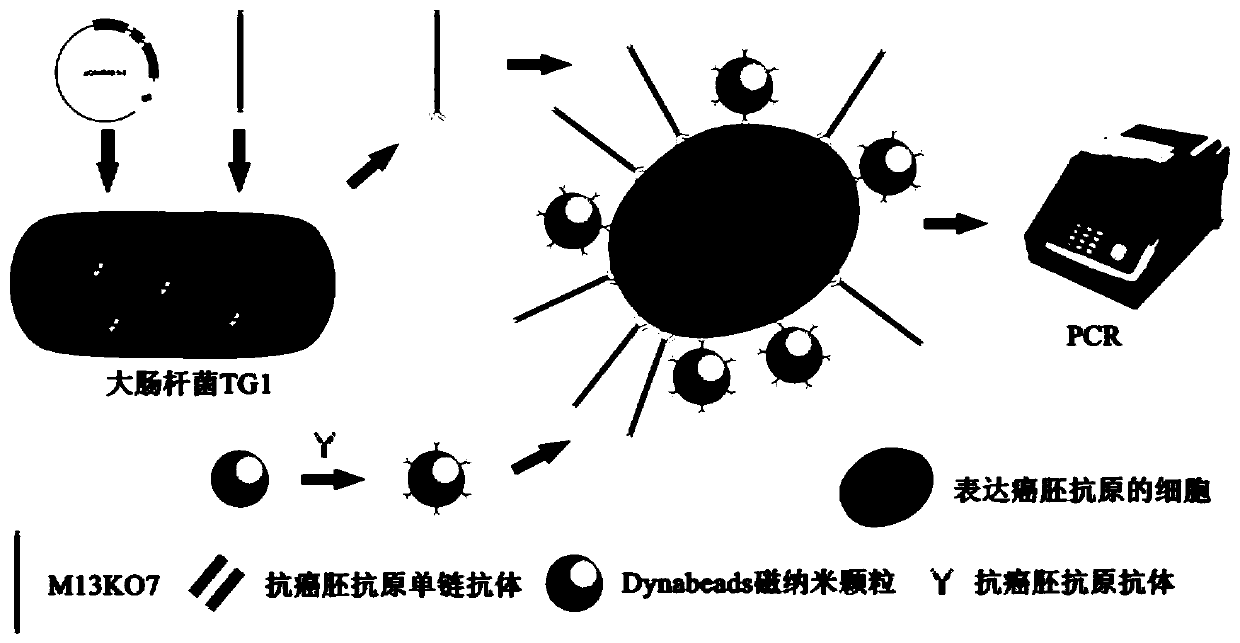
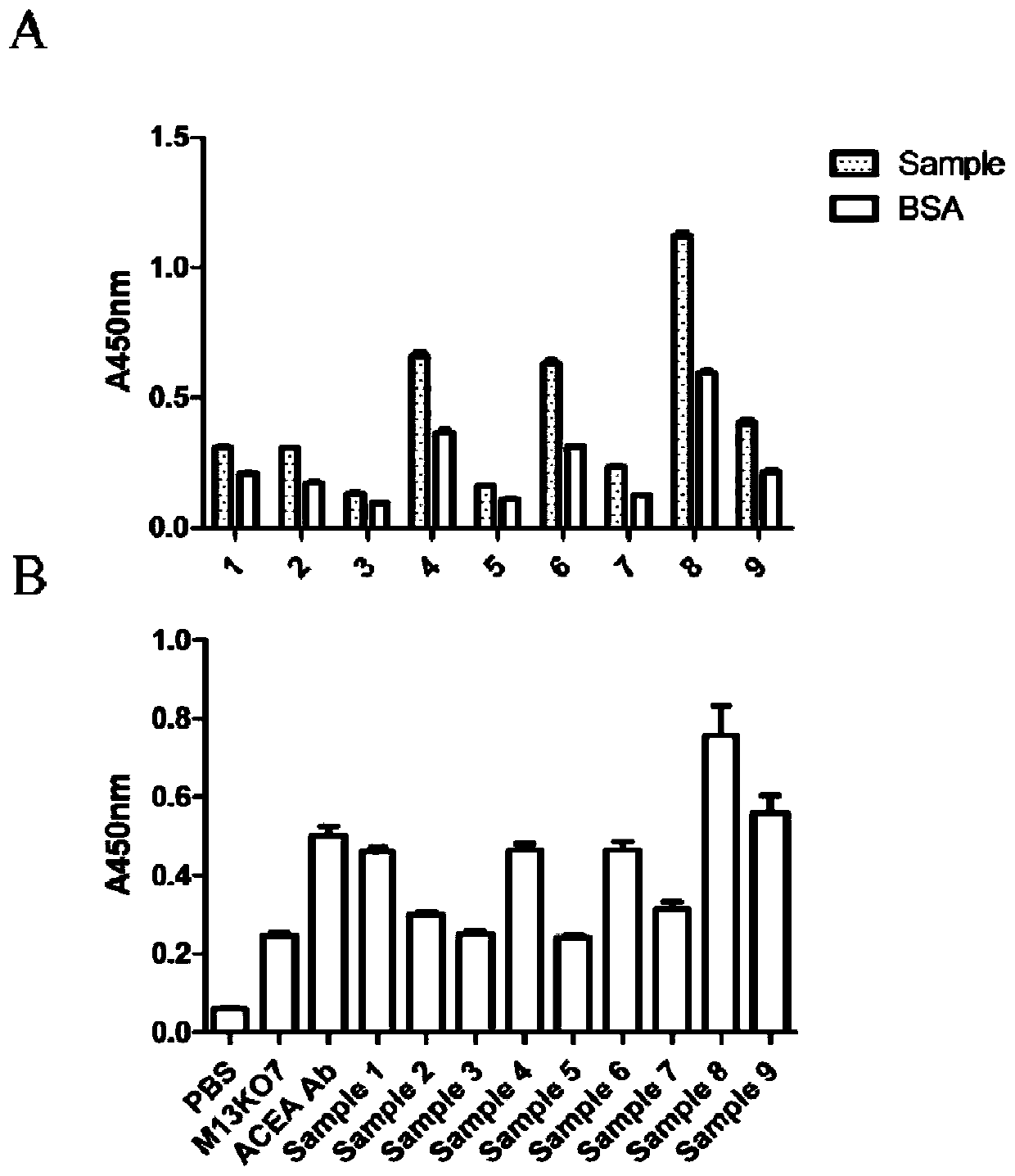
Method for detecting cell capable of expressing carcinoembryonic antigen, and application thereof
InactiveCN110231484AQuick checkSensitive detectionMaterial analysisSingle-Chain AntibodiesMagnetite Nanoparticles
The invention relates to a method for detecting a cell capable of expressing a carcinoembryonic antigen, and application thereof. A bacteriophage display technology is used for displaying a single chain antibody capable of resisting the carcinoembryonic antigen on the surface of auxiliary bacteriophage M13KO7 to construct a library of the single chain antibody capable of resisting the carcinoembryonic antigen. The library is used as a probe, the cell capable of expressing the carcinoembryonic antigen is subjected to specific targeted combination, and then, a magnetic nanoparticle modified by an antibody capable of resisting the carcinoembryonic antigen is used for capturing the category of cells. Through PCR (Polymerase Chain Reaction), the g3p gene of M13KO7 is amplified so as to achievea purpose of quickly and sensitively detecting the single cell capable of expressing the carcinoembryonic antigen or low-content circulating tumor cells.
Owner:YANGZHOU UNIV
A method for preparing nanobodies against carcinoembryonic antigen
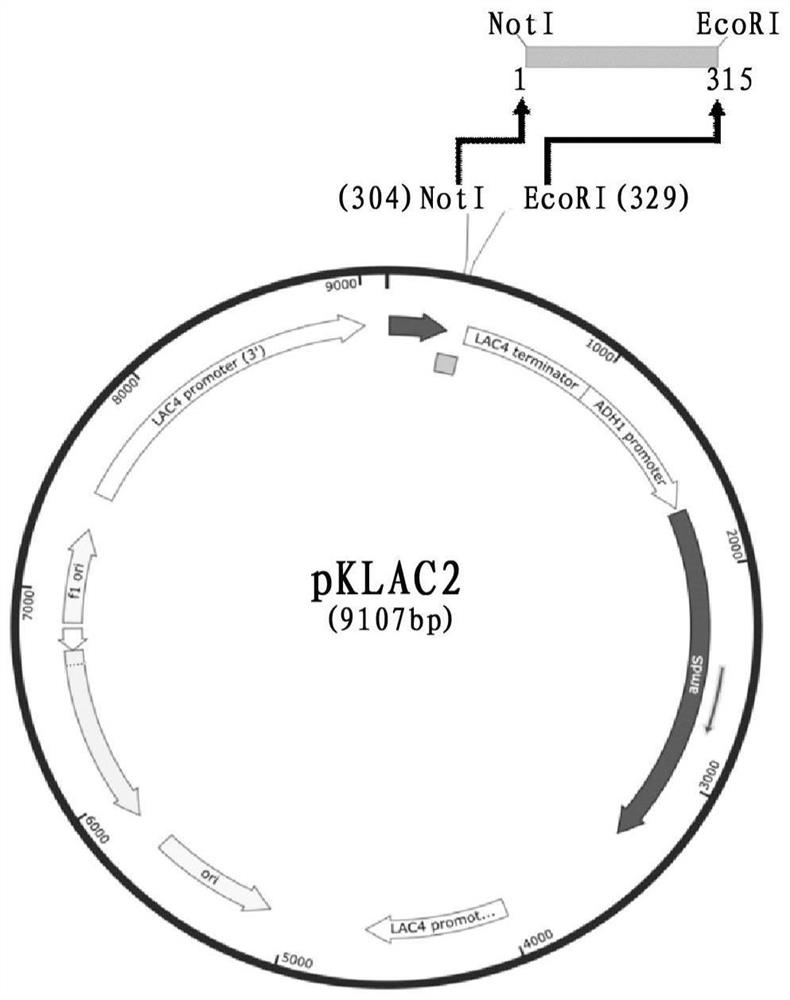
ActiveCN108103068BSimple methodEfficient methodFungiMicroorganism based processesForeign proteinExtracellular
The invention provides a method for preparing a nanobody against carcinoembryonic antigen, said method comprising the following steps: (1) amplifying the coding gene of the carcinoembryonic antigen nanobody; (2) converting the coding gene obtained in step (1) to Linked to the expression vector pKLAC2; (3) transducing the vector obtained in step (2) into the host cell Kluyveromyces lactis; (4) inducing the host cell obtained in step (3), and harvesting the expressed anti-carcinoembryonic antigen nano Antibody. The invention also provides an expression vector containing the anti-carcinoembryonic antigen nanobody and a host cell Kluyveromyces lactis containing the vector. Using the method of the present invention, the expressed nanobody is secreted to the outside of the cell, the foreign protein is less, and the target band detected by Western Blot is single, thereby reducing the cost of separation and purification, and greatly improving the efficiency of expression; Under the conditions of bottle culture, the amount of extracellular secretion was 0.8 mg / l, and the affinity of the nanobody against carcinoembryonic antigen measured by direct coupling method was 5.042×10 ‑9 .
Owner:深圳市国创纳米抗体技术有限公司
Blood routine spectroscopic isotope effect technology for tumor detection
InactiveCN106153564AHigh sensitivityStrong detection and grading performanceColor/spectral properties measurementsUltravioletIsotope
A blood routine spectroscopic isotope effect technology for tumor detection has the following two definitions: for one definition, a one-dimensional blood routine ultraviolet spectrogram (ordinary ultraviolet spectrum) is adopted for detection, and P53 fluorescent sequencing and carcinoembryonic antigen detection belong to the method; for the other definition, a two-dimensional ultraviolet spectrogram analysis technology provides excited-state information of interaction between DNA and protein, and provides gene expression information which cannot be obtained with a large number of one-dimensional methods. 90% of early-stage cancer is discovered through professional general surveys, and regular cancer screening is still the best way for humans to defend against cancer. By the aid of matching of one-blood-drop tumor blood routine spectroscopic isotope effect technology (two-dimensional blood routine excited-state information) with the carcinoembryonic antigen detection (one-dimensional blood routine enzyme-linked immunoabsorbent assay) technology, genetic molecule expression and electronic level expression can be screened accurately and rapidly, and great significance is provided for early diagnosis and treatment of tumors. The blood routine spectroscopic isotope technology is quite suitable for universal screening of the tumors with only one drop of blood, and the effects of early discovery and early diagnosis are realized.
Owner:王汉成
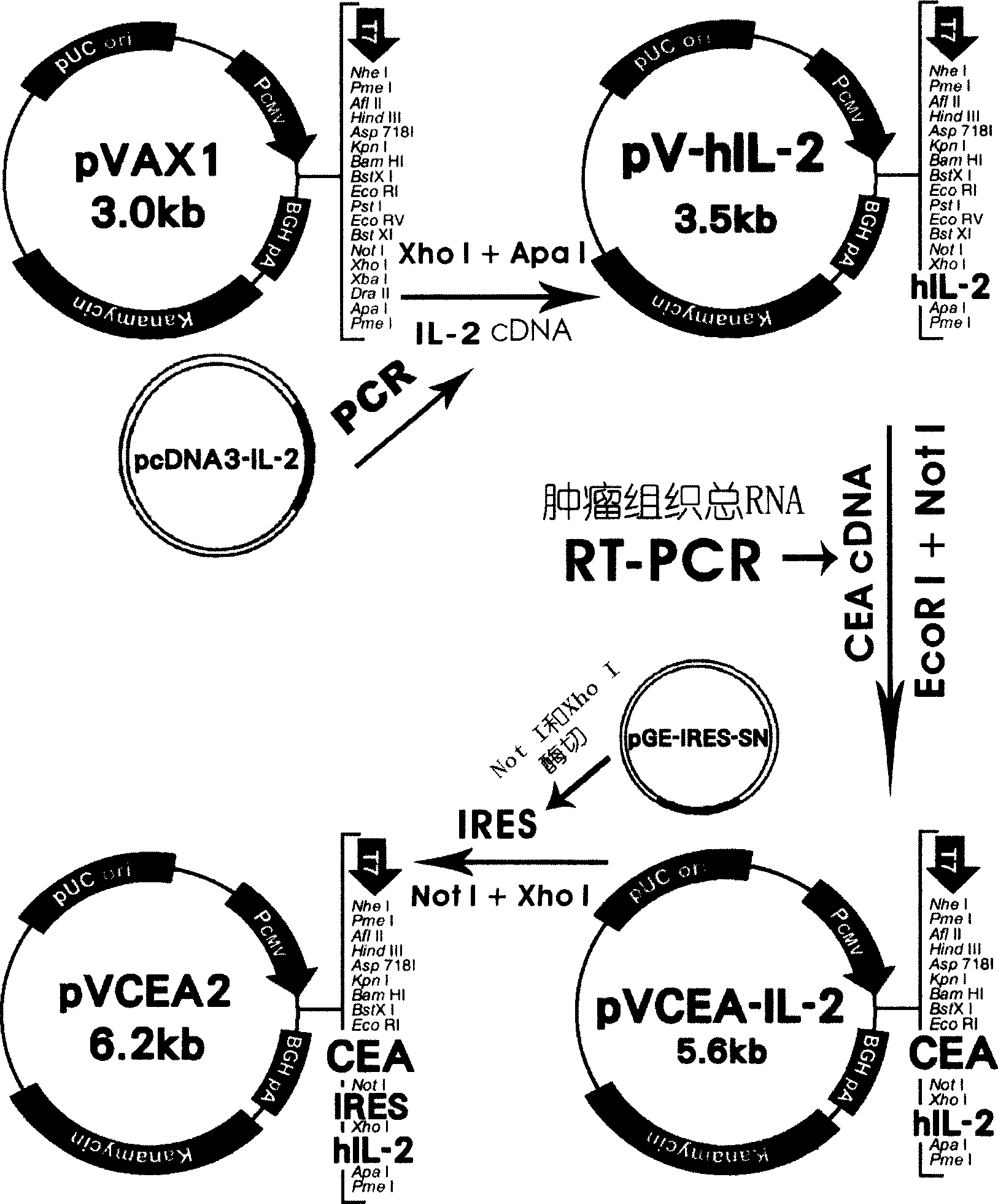
CCA gene vaccine, and its establishing method and use for preparing vaccine for preventing and treating tumour
ActiveCN1611264ANo immunotoxicityApparent levelGenetic material ingredientsAntibody medical ingredientsEucaryotic cellCarcinoembryonic Antigen Positive
The present invention relates to a CEA gene vaccine, its construction method and application in preparation of vaccine for preventing and curing tumor. It is characterized by that it utilizes eukaryotic cell expression plasmid to construct the carcion-embryonic antigen (CEA) gene vaccine with interleukin 2 coordinate expression. The detection shows that in the external cell it can implement coordinate expression of CEA and IL-2 protein molecule, and the animal model tests show that the CEA gene vaccine has the action of preventing and curing carcion-embryonic antigen positive tumor, and in the body it has not immunotoxic effect.
Owner:中山大学中山医学院科技开发中心 +1
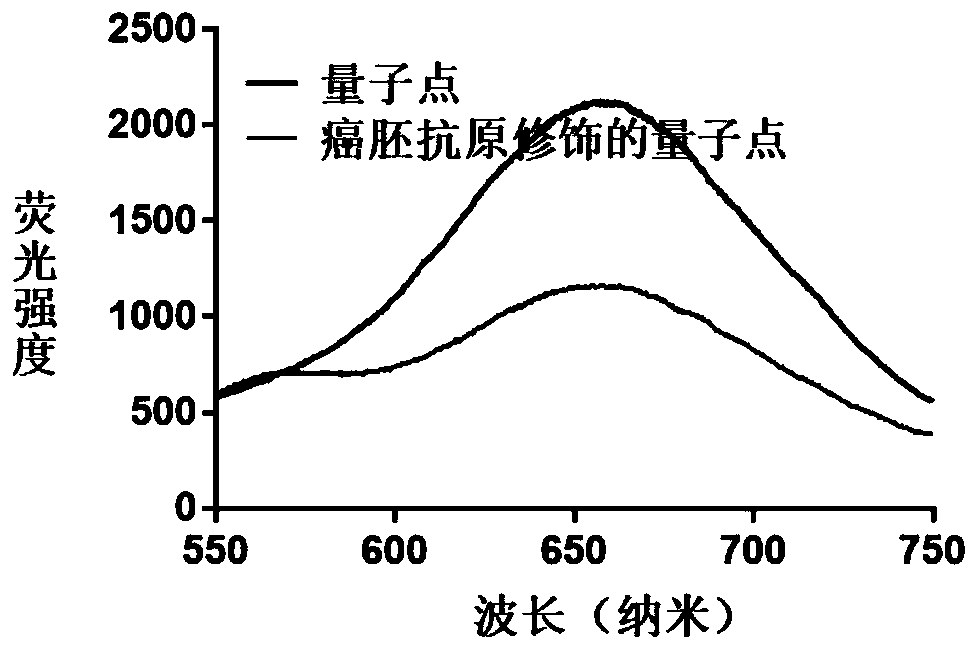
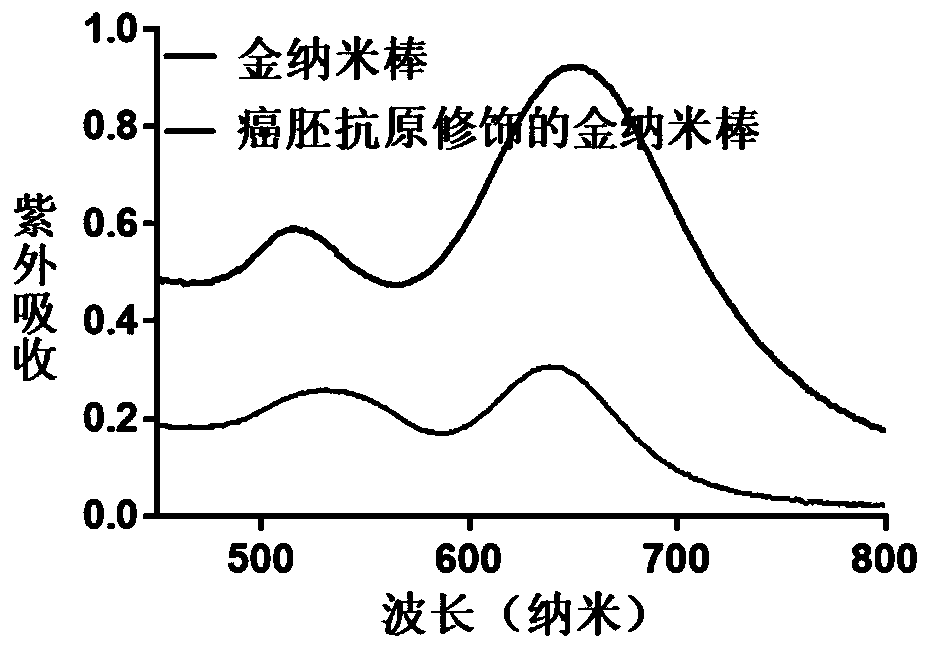
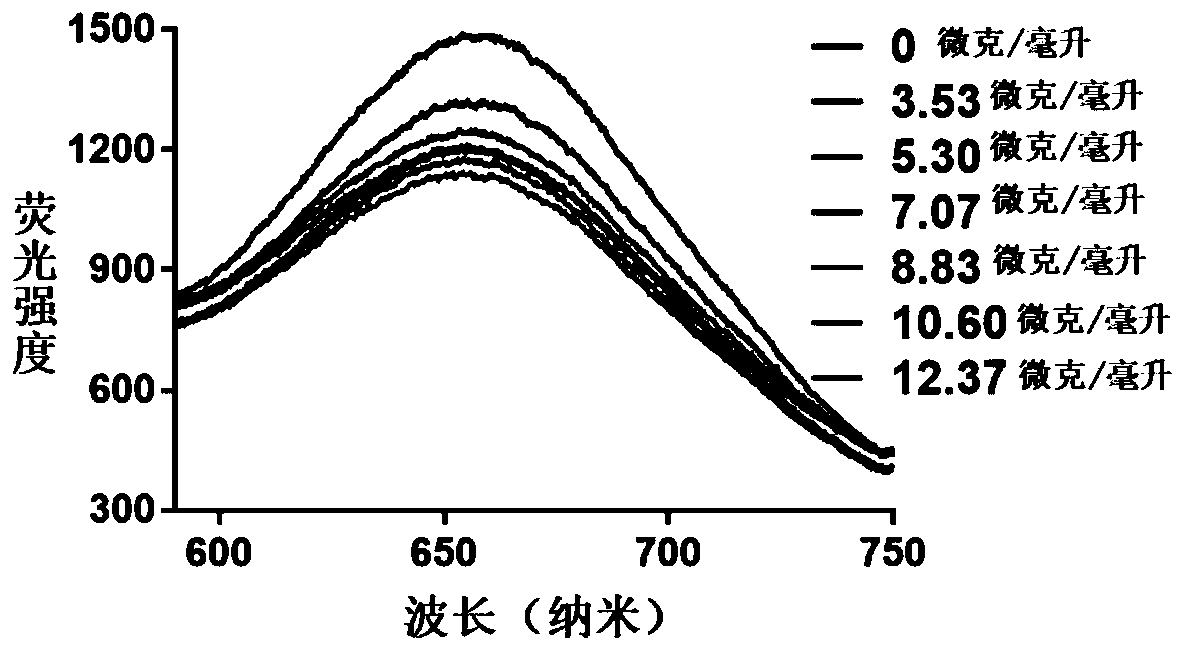
Hydrogel preparation based on quantum dots and gold nanorods as well as preparation method and application of hydrogel preparation
The invention provides a preparation method of a hydrogel preparation based on quantum dots and gold nanorods. The preparation method comprises the following steps of firstly, combining the quantum dots with a carcinoembryonic antigen aptamer; secondly, combining the gold nanorods with the carcinoembryonic antigen aptamer; thirdly, mixing the quantum dots modified by the carcinoembryonic antigen aptamer, the gold nanorods modified by the carcinoembryonic antigen aptamer and a hydrogel solution; and finally, obtaining the hydrogel preparation based on the quantum dots and the gold nanorods. Theprepared hydrogel preparation can be used for detecting a carcinoembryonic antigen, and when the hydrogel preparation is co-incubated with the carcinoembryonic antigen whose concentration of 1-6 microliters is 1 mg / ml, the light quenching efficiency is 12-24%.
Owner:TIANJIN UNIV
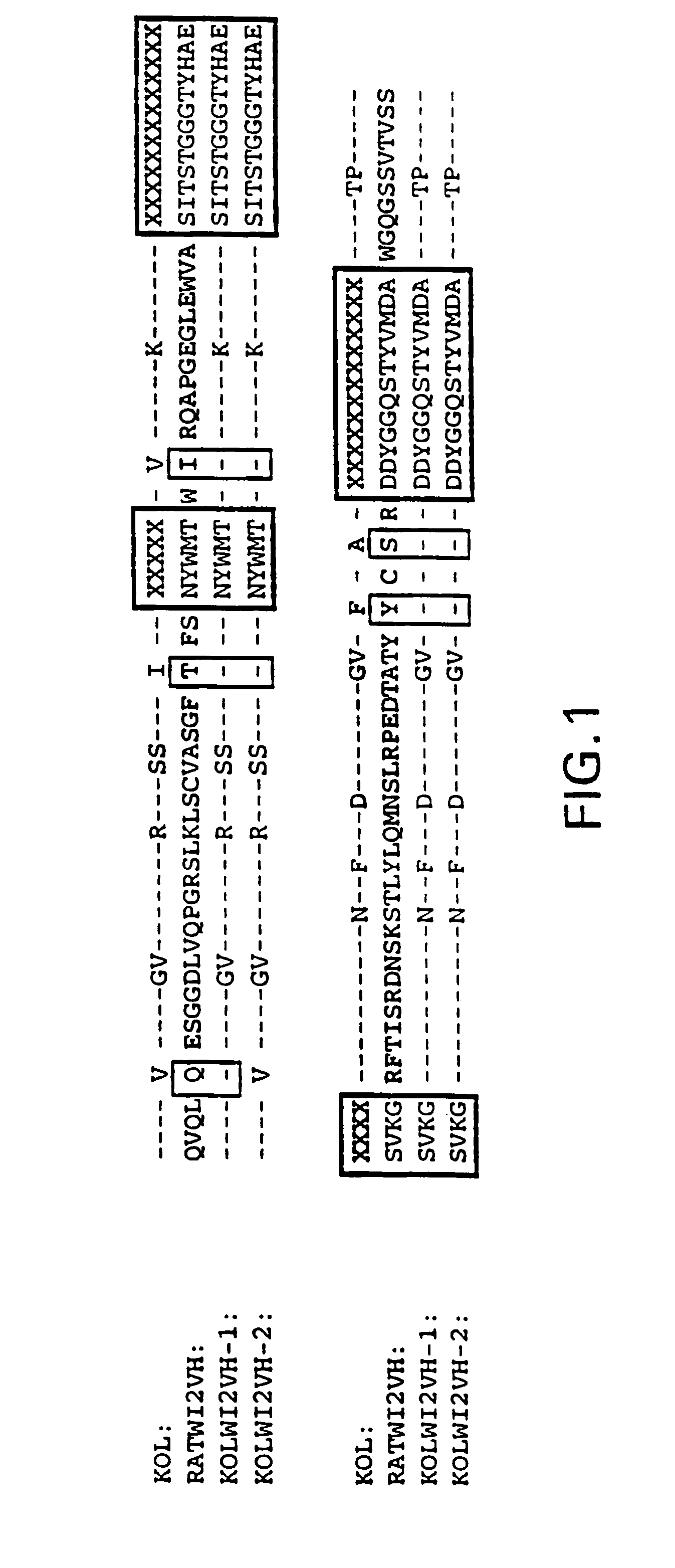
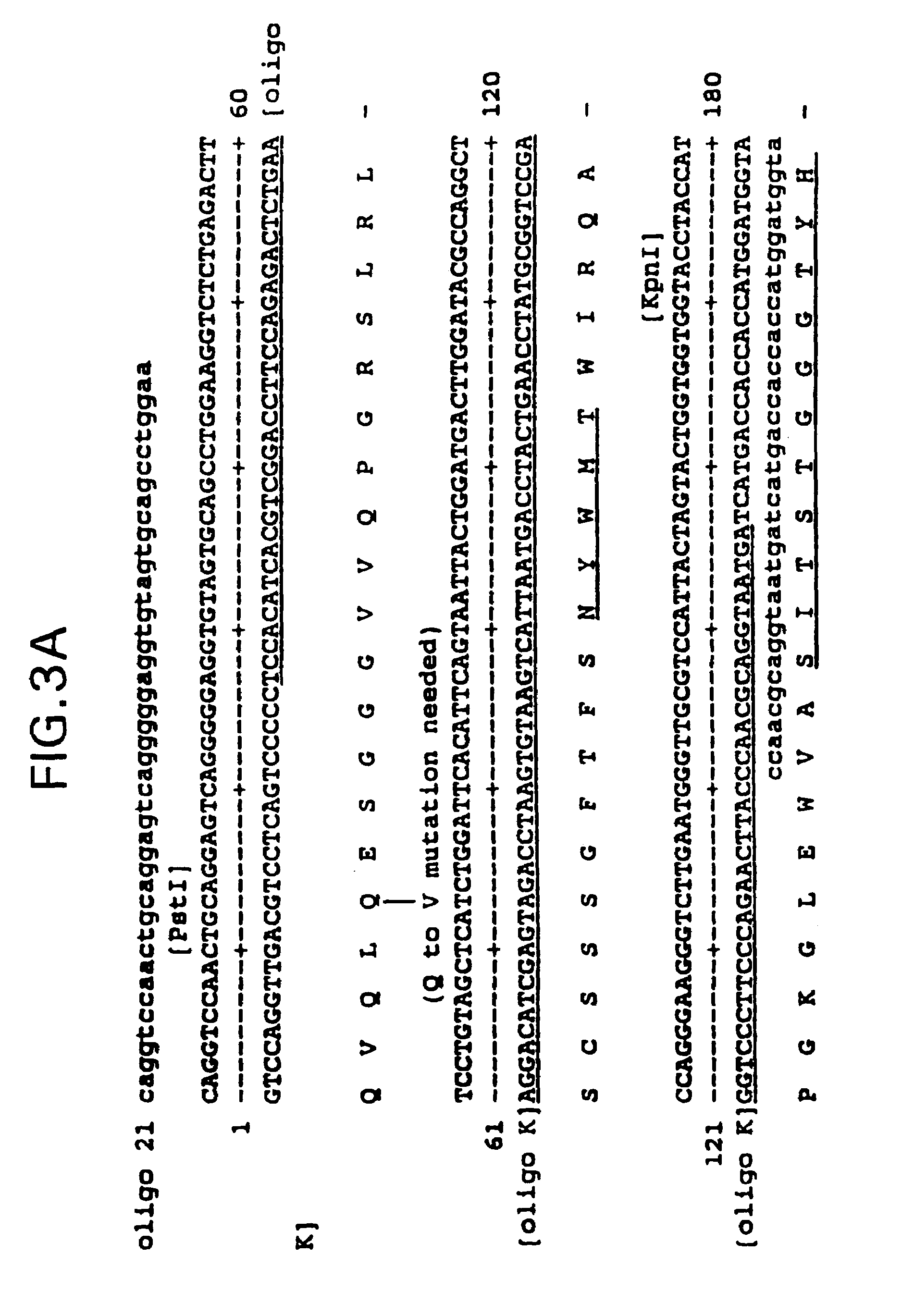
Humanization of an anti-carcinoembryonic antigen anti-idiotype antibody as a tumor vaccine and for targeting applications
A humanized form of an anti-idiotype antibody to CEA, e.g., hWI2, has conserved immunoreactivity. The clinical benefits of anti-CEA antibodies are maximized by using the humanized anti-idiotype as a clearing agent for anti-CEA antibodies or antibody fragments. The humanized anti-idiotype also can be used as an immunogenic vaccine.
Owner:IMMUNOMEDICS INC
An electrochemiluminescence multi-component immunoassay method based on the principle of spectral resolution
ActiveCN106124487BReduce consumptionShorten the timeChemiluminescene/bioluminescenceBiological testingAntigenCarcinoembryonic Antigen Positive
The present invention relates to an electrochemiluminescence multicomponent immunodetection method based on a spectral resolution principle, wherein CdSe quantum dots and CdTe quantum dots are adopted as markers, and the ECL signals of different markers are identified based on a spectral resolution principle so as to provide the ECL multicomponent detection method. The method mainly comprises: (1) preparing three quantum dot labeled corresponding secondary antibody (QDs-Ab2); (2) preparing a multicomponent ECL immunosensor using the three quantum dots as markers; and (3) carrying out multicomponent ECL immunodetection. According to the present invention, the detection sensitivity of the method is high, the detection limit of the carcinoembryonic antigen achieves 1.0 pg / mL, the detection limit of the prostate specific antigen achieves 10.0 pg / mL, the detection limit of the alpha fetoprotein antigen achieves 10.0 fg / mL, the concurrent detection of a variety of antigens can be achieved, and the selectivity is high.
Owner:SHANDONG UNIV
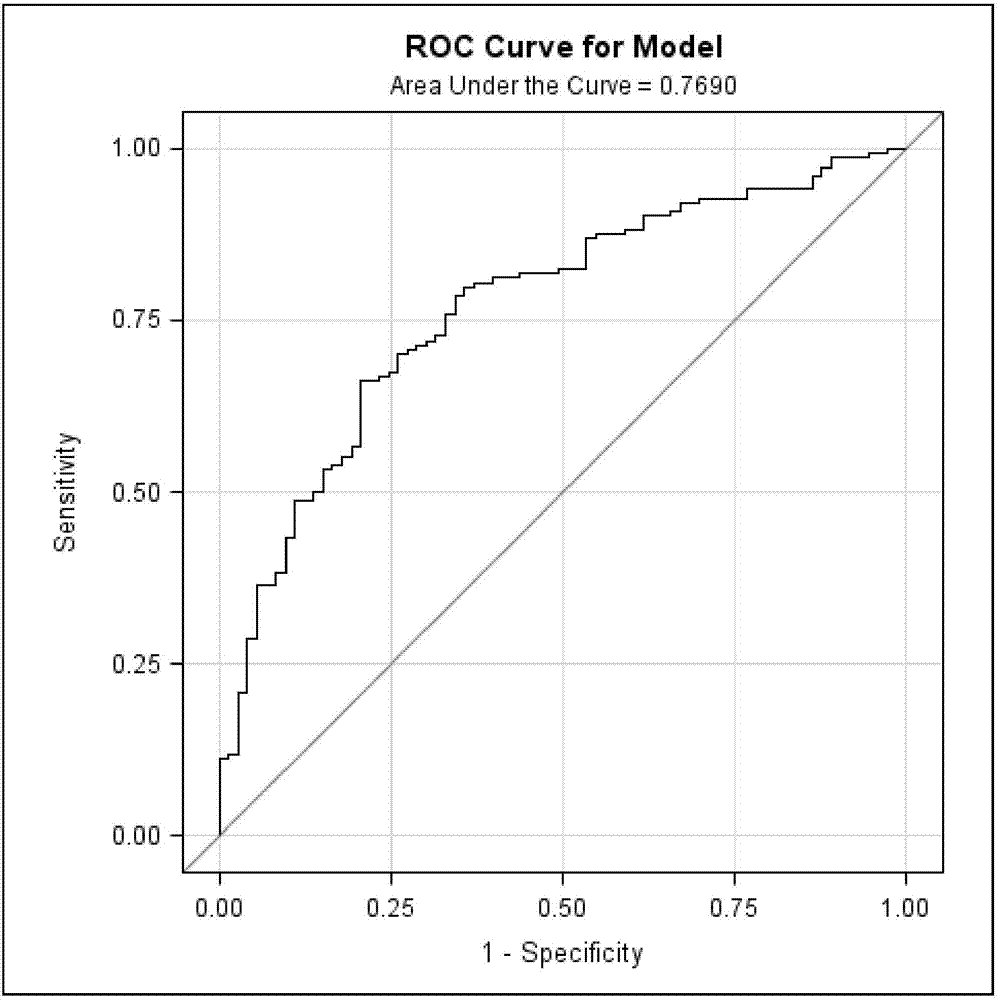
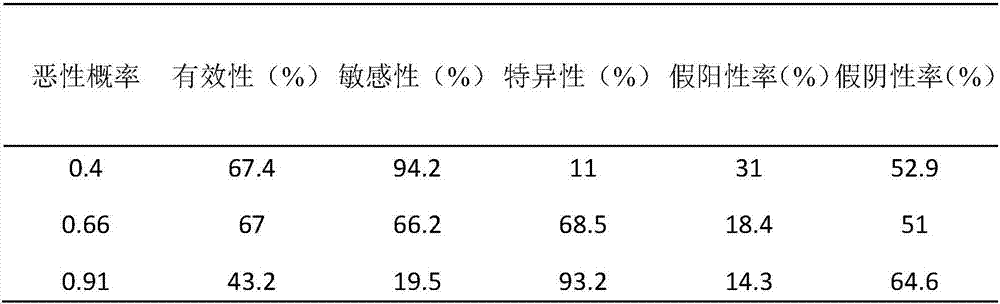
A lung cancer risk prediction model based on biomarker profiles for pulmonary nodules in rural China
ActiveCN105891482BEasy to measureDetermination is accurate and reliableMaterial analysis by observing effect on chemical indicatorPulmonary noduleAntigen
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com