CCA gene vaccine, and its establishing method and use for preparing vaccine for preventing and treating tumour
A gene vaccine and tumor vaccine technology, applied in gene therapy, antitumor drugs, pharmaceutical formulations, etc., can solve problems such as unsatisfactory effects, killing tumors, and inability to achieve specificity, and achieve good preventive effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
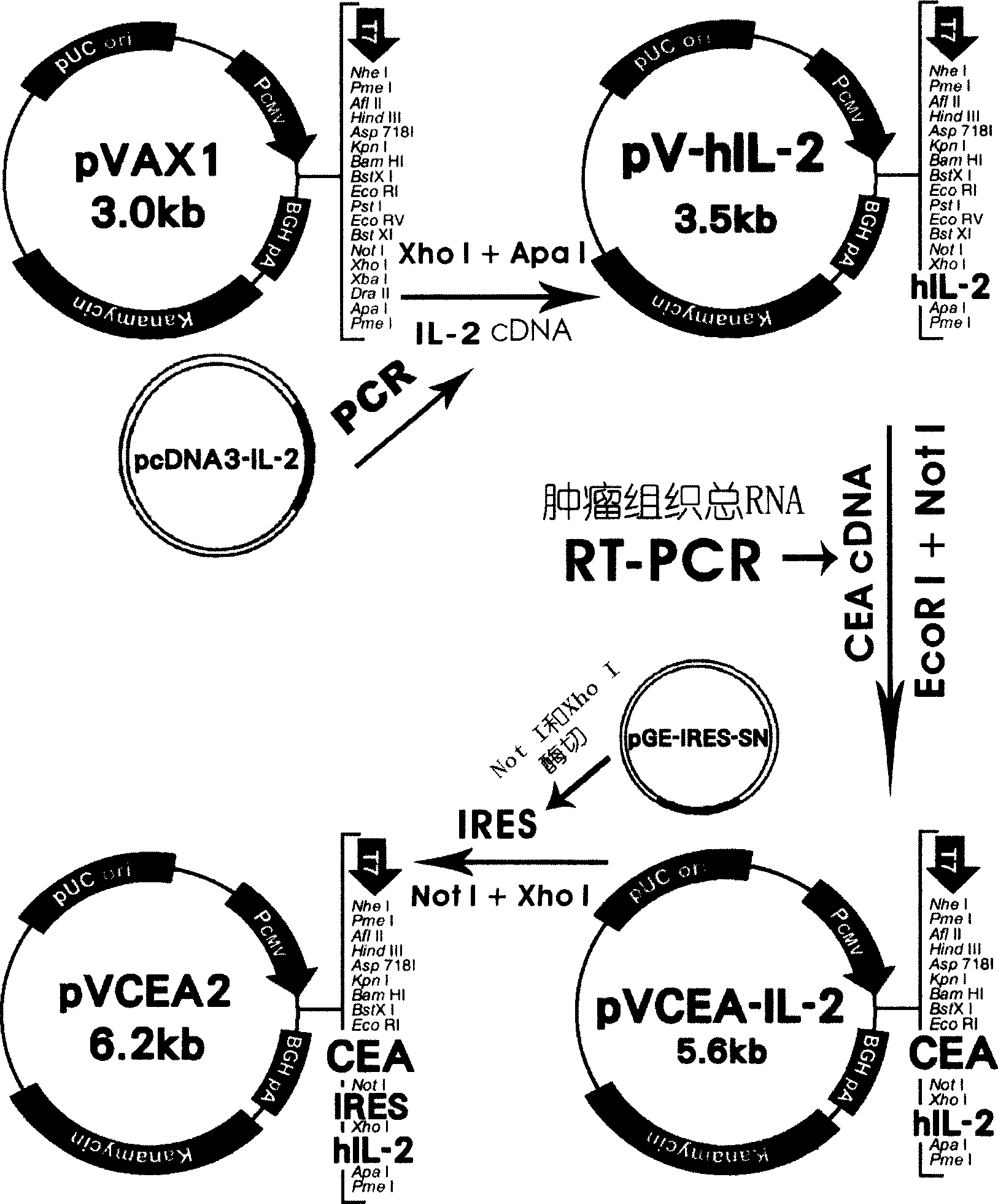
[0018] 1.1 Extraction and identification of total RNA from CEA tumor tissue: according to Invitrogen Concert TM Cytoplasmic RNA Reagent is a one-step method for extracting total cellular RNA. The total RNA extract was identified by 1% formaldehyde gel electrophoresis, and the OD was measured with a UV spectrophotometer 260 and OD 280 value and stored at -70°C for future use.
[0019] 1.2 Primer synthesis and RT-PCR amplification of cDNA encoding human CEA: The full-length cDNA sequence of M17303 published by GenBank was used as a template for primer design, and EcoR I and Not I restriction endonuclease sites were introduced respectively, and the GeneFisher online Screen and analyze the GC ratio, Tm value, hairpin structure, motif structure and specificity of the designed PCR primers through molecular biology software PCRDESN and Oligo5.0, and synthesize the following primers:
[0020] Sense primer: 5'GTCGAATTCAGACAGCAGAGACCATGGA-3'
[0021] Antisense primer: 5'AAGCGGCCGCA...
Embodiment 2
[0058] Example 2 After in vitro transfection with CHO (Chinese hamster ovary cell line), the expression of CEA antigen and hIL-2 was detected
[0059] 2.1 CHO cell recovery, culture, and liposome transfection CHO cells taken out of liquid nitrogen were quickly placed in a 37°C water bath to thaw and recover, centrifuged at 2000rpm for 5min, discarded the supernatant, and added RPMI 1640 cells containing 10% newborn calf serum Medium (without antibiotics), 37°C and 5% CO 2 cultivated in. Digest the adherent monolayer cells that grow to more than 85% with 0.025% trypsin, add the culture medium and blow the cells, centrifuge at 1000rpm for 10min, count the cells and pass them in a 6-well plate for 24h after dilution, and then add about 4μg of the recombinant plasmid pVCEA2 (mixed in 250 μl of serum-free medium) and 10 μl of lipofectAMINE2000 (mixed in 250 μl of serum-free medium) were gently mixed and transfected into CHO cells. At the 24th and 48th hour, 200 μl of the culture ...
Embodiment 3 Embodiment 1
[0072] The CEA antibody level detection of the immunized mice of the CEA gene vaccine of embodiment 3 embodiment 1
[0073] 3.1 Experimental method: Secondary male BALB / C mice, 6-8 weeks old, were randomly divided into five groups, with 6 mice in each group.
[0074] I pVAX1 control group
[0075] II pVCEA experimental group (pVAX1-CEA-hIL-2)
[0076] III pVCEA2 experimental group (i.e. the genetic vaccine that embodiment 1 obtains)
[0077] IV mixed experimental group (CEA-rV+pVCEA2)
[0078] V compound experimental group (pVCEA2+pV35-I-40)
[0079] Before each immunization of the mice, inject 0.25% bupivacaine into each 50 μl of the quadriceps femoris of the two hind legs, and then inject 50 μg of the plasmid on each side after 24 hours (the medicine for the first immunization injection in the mixed group is CEA-rV, Dosage is 5×10 6 PFU, pVCEA2 for the second and third times; 50 μg for each component in the compound group). On the 25th day and the 45th day, they were i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com