Preparation and application of vaccine for curing tumor in positive carcino-embryonic antigen
A carcinoembryonic antigen and element technology, applied in the fields of biology and medicine, can solve the problem of no CEA-positive tumor therapeutic vaccine and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
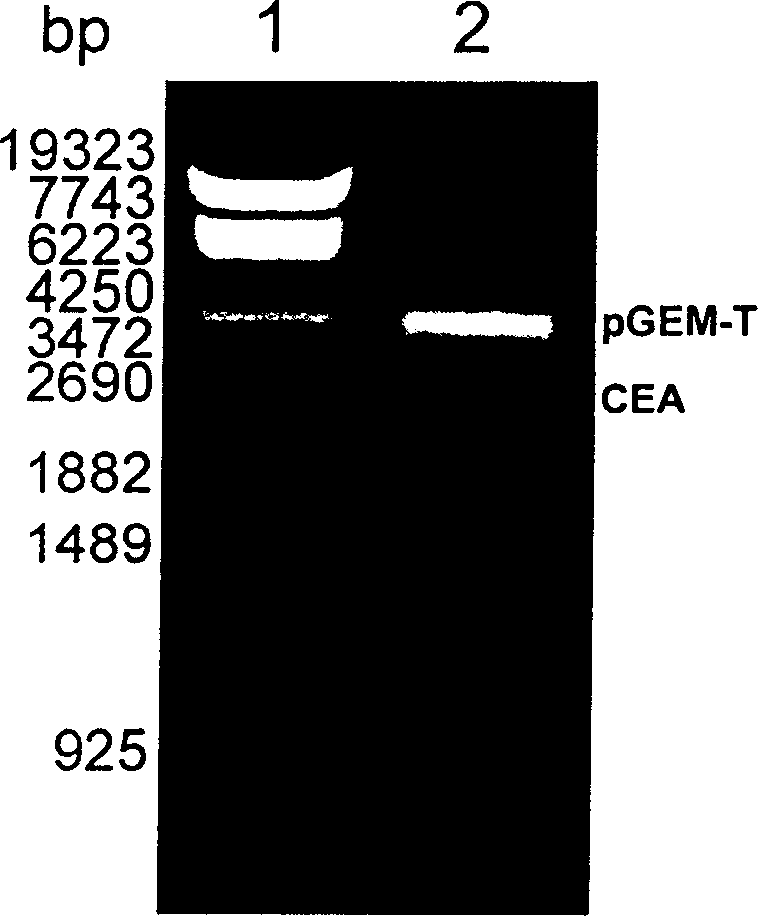
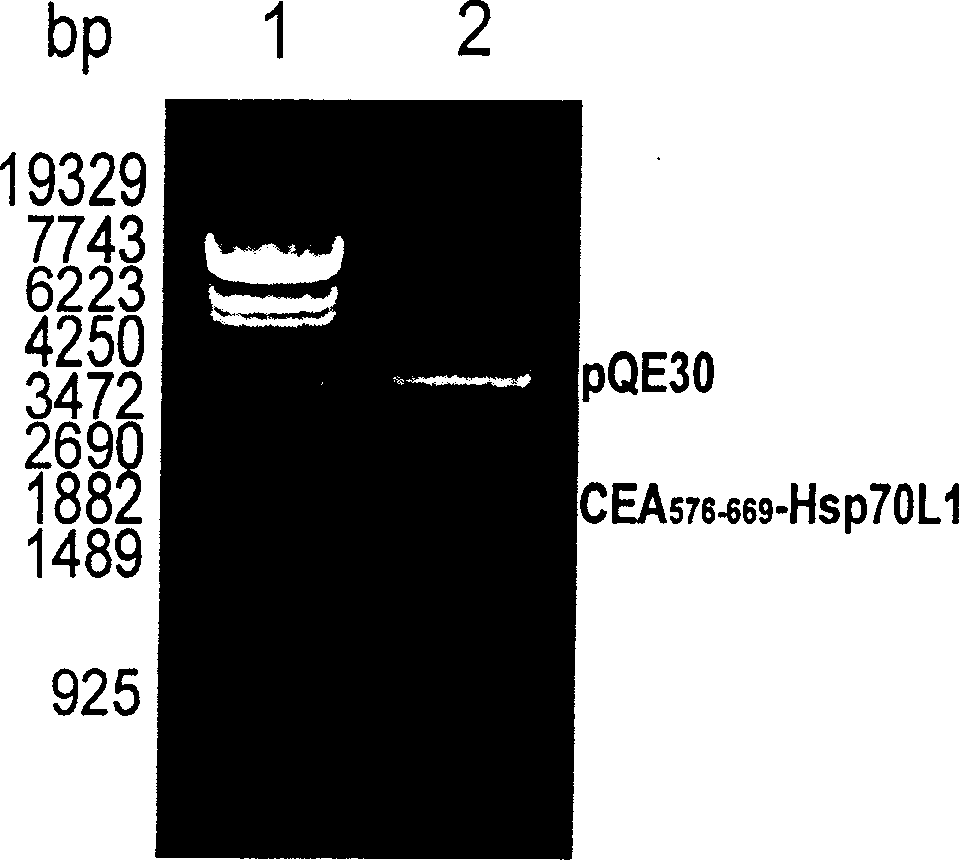
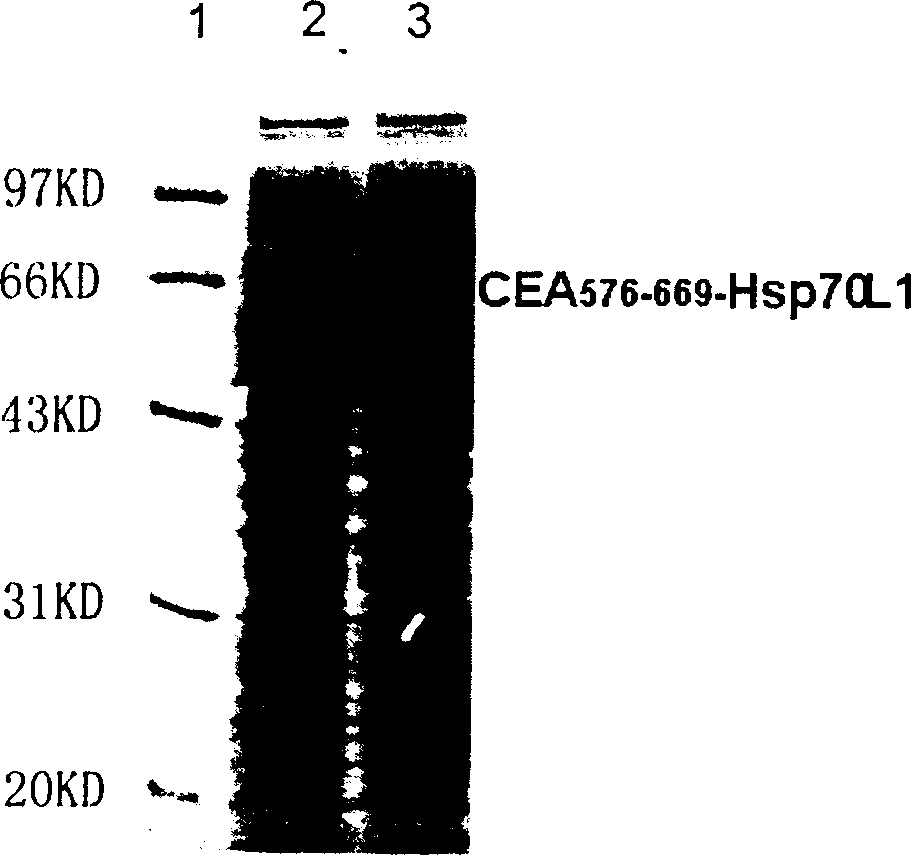
[0073] Example 1 Recombinant CEA 576-669 -Expression and purification of Hsp70L1 protein
[0074] 1.1 Cloning of human CEA cDNA:
[0075] Collect CEA-positive human colon cancer cells LS 174T (ATCC Number: CL-188), use total cell RNA extraction reagent Trizol (Invitrogen Company) to prepare cell total RNA, use AMV reverse transcriptase (Promega Company) to synthesize the first strand, and It is used as a template, and PCR oligonucleotide primers with the following sequences at the 5' and 3' ends are used to amplify to obtain a DNA fragment encoding CEA.
[0076] The 5' end oligonucleotide primer sequence used in the PCR reaction is:
[0077] 5'-CACGGTACCACCATGGAGTCTCCCTCGGCCCC-3' (SEQ ID NO: 1)
[0078] The primer contains an enzyme cutting site of KpnI restriction endonuclease, and behind the enzyme cutting site is a partial coding sequence of a translation initiator and CEA;
[0079] The 3' end primer sequence is:
[0080] 5'-CTCGTCGACTATCAGAGCAACCCCAACCAG-3' (SEQ ID NO...
Embodiment 2
[0116] Example 2 Preparation of a new therapeutic dendritic cell vaccine for CEA-positive tumors
[0117] 2.1 Isolation and culture of DC and T lymphocytes:
[0118] Take freshly isolated colon cancer patients (CEA + , HLA-A*0201 + ), peripheral blood mononuclear cells were separated by density gradient centrifugation of lymphocyte separation medium (Ficoll-Histopaque 1.077) (Sigma Company). Set at 37°C, 5% CO 2 Incubate the adherent monocytes for 2 hours in complete medium containing human recombinant GM-CSF (500 U / ml) (Schering Bauer) and human recombinant IL-4 (10 ng / ml) (Promega) 37 °C, 5% CO 2 Culture was carried out to obtain DCs derived from peripheral blood mononuclear cells. The non-adherent cells were suspended in RPMI 1640 medium with 5% fetal bovine serum, passed through a nylon wool column (incubated at 37° C. for 1 hour), and purified T lymphocytes were obtained.
[0119] 2.2 Sensitization of DC:
[0120] Collect DCs derived from peripheral blood mononucle...
Embodiment 3
[0122] Example 3 CEA 576-669 -Hsp70L1 fusion protein sensitized dendritic cells to induce CEA-specific T lymphocytes in vitro
[0123] 3.1 CTL killing assay:
[0124] 4 hours 51 The killing ability of CTL was detected by Cr release method. With SW480 cells (CEA + , HLA-A*0201 + ), LoVo cells (CEA + , HLA-A*0201 - ), T2 cells were used as target cells, respectively. Part of the T2 cells were suspended in 200 μl RPMI1640 medium without fetal bovine serum, and first mixed with 10 μg / ml CAP-1 (CEA 605-613 , YLSGANLNL, for CEA 576-669 An HLA-A*0201-restricted epitope contained in ) or an unrelated peptide Tyr 368-376 Incubate for 60 minutes. Add 200μCi 51 Cr (Amersham Pharmacia Company), placed in a 37°C water bath for 90 minutes, mixed gently every 15 minutes, and then washed thoroughly with medium to remove residual 51 Cr, used as the target cell for killing, was added to the corresponding prepared effector cells (CD8+ T cells from each group) according to three diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com