Integrated test reaction plate of five indicators of prenatal and postnatal care and kit
A detection kit and integrated detection technology, applied in the field of in vitro clinical testing and biochips, can solve the problems of laboratory personnel's health threat, complex composition, large sample size, etc., to improve detection speed and efficiency, simple production process, and monitoring results. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of an integrated detection reaction plate for five indicators of prenatal and postnatal care
[0041] The main steps are: (1) Prepare the matrix nitrocellulose membrane; (2) Design the chip, determine the arrangement of the lattice and the spotting position; (3) Draw the antibody from the 384-well plate and spray it on the nitrocellulose (4) assemble a nitrocellulose membrane, and carry out sealing, drying, packaging, and preservation.
[0042] The above reaction plate preparation method for detecting the five indicators of prenatal and postnatal care specifically includes the following steps:
[0043] 1. Determination of spotting antigens for five indicators related to prenatal and postnatal testing:
[0044] Toxoplasma gondii Antigen (Strain RH), Rubella Antigen (HPV-77), Cytomegalovirus Ag (AD169), Herpes Simplex Virus I (HSV1 ) and Herpes Simplex Virus II (HSV2 whole virus antigens) were purchased from BioDesign.
[0045] Determination of enzyme-labele...
Embodiment 2
[0054] Preparation of integrated detection kit for five indicators of prenatal and postnatal care
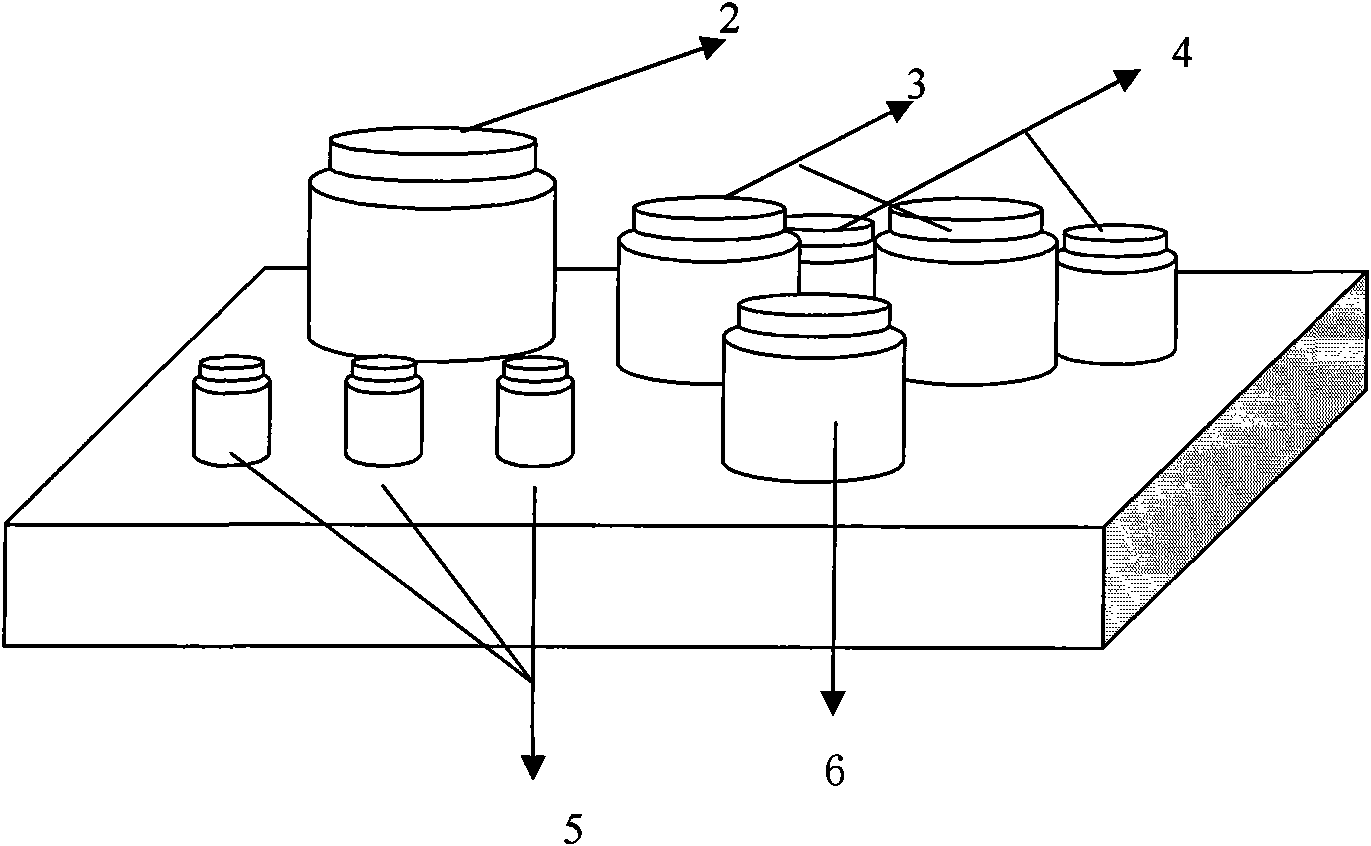
[0055] Including integrated detection reaction plate for five indicators of prenatal and postnatal care, negative control substance, positive control substance I, II, sample diluent, enzyme-labeled working solution I, II, concentrated washing solution, detection solution A, B; each part is bottled, Fix it in the box with a paper template (such as image 3 ), not limited to the placement method, it is used together with the test. Here's how the parts are prepared:
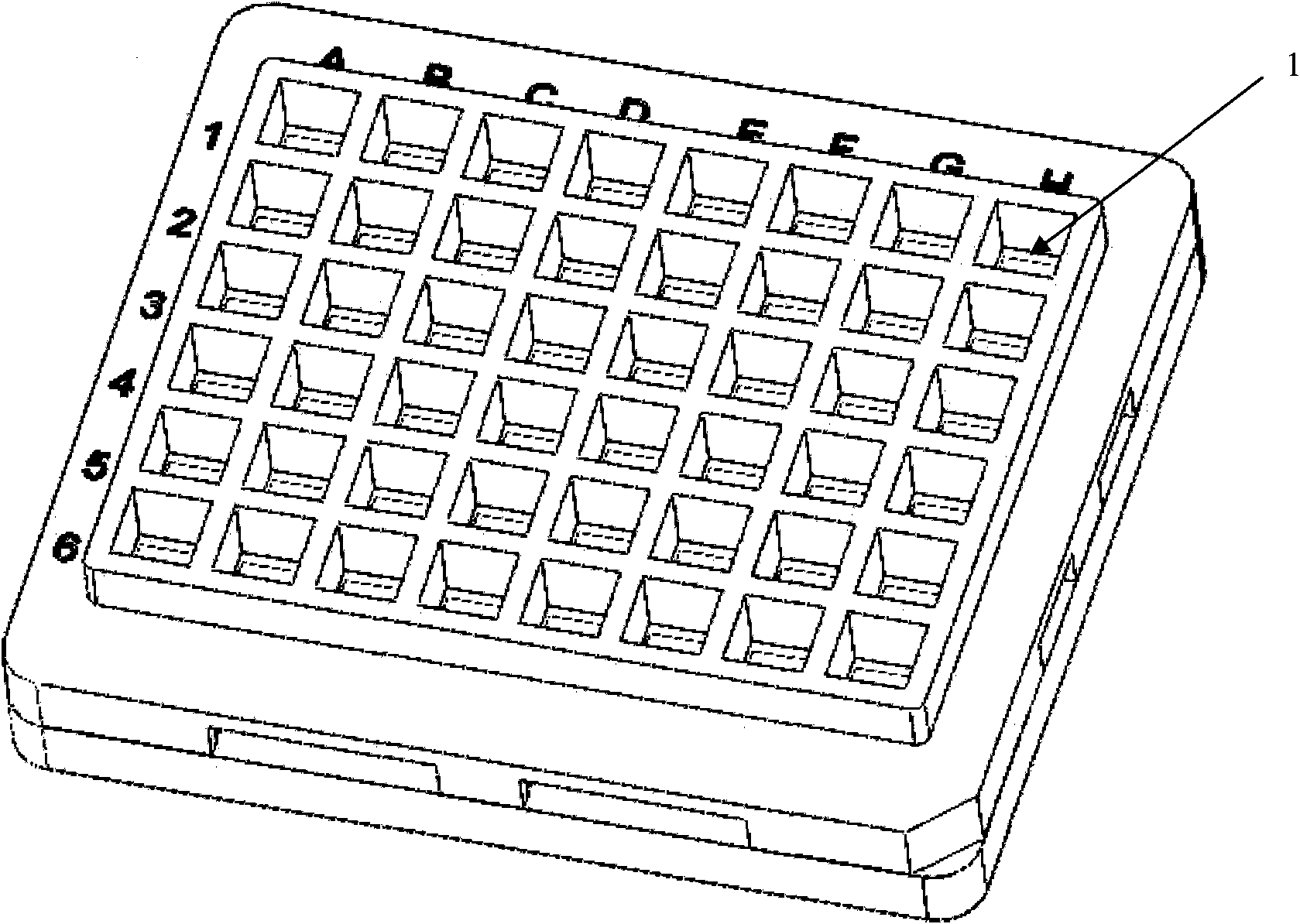
[0056] 1. The prenatal and postnatal detection protein chip is a 6×8 well plate, with nitrocellulose membrane as the base, and the assembly method is shown in ZL032298900. After the completion of the appearance, see figure 1 .
[0057] 2. Provide 1 bottle of negative control 5, 0.4ml per bottle;
[0058] Prepared in phosphate buffered saline (pH 7.4) with 3% BSA and 0.05% preservative (ProclinTM 300). Provides 1...
Embodiment 3
[0071] Application and detection of integrated detection kit for five indicators of prenatal and postnatal care
[0072] The application of the integrated detection kit for detecting the five indicators of prenatal and postnatal care according to the present invention mainly uses the method of antigen-antibody-enzyme-labeled secondary antibody to detect the five prenatal and postnatal care antibodies in human blood. The method is to add human serum to the surface of the chip, and the antibodies in the sample are respectively combined with the corresponding antigens immobilized on the chip. After drying, add the enzyme-labeled secondary antibody to react, wash away the unbound secondary antibody, and add the detection solution after 1 Minutes, scan and collect the signal, the signal intensity of the sample point is proportional to the concentration of the antibody in the serum.
[0073] In the application of the above-mentioned protein chip kit for detecting the five items of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com