Cloning and analysis of endo-beta-1,4-glucanase(Aus cel5A) gene
An endoglucanase and gene technology, applied in genetic engineering, plant genetic improvement, enzymes, etc., can solve problems that have not been reported before, and achieve the effect of large-scale industrial production and application potential and economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
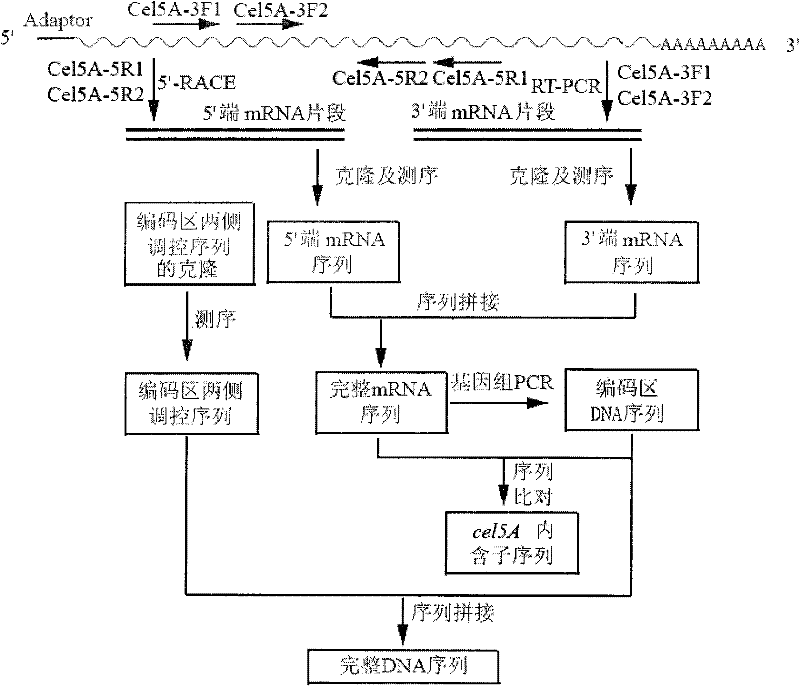
[0030]Cloning of Example 1 Aus cel5A 3' end mRNA sequence
[0031] The first strand of cDNA was synthesized by reverse transcription with Oligo dT-Adaptor Primer as primers; the first round of PCR was performed with M13 Primer M4 and Cel5A-3F1 as primers, and the reaction conditions were: 94°C for 2min, 30 cycles (94°C 30s, 52°C for 30s, 72°C for 90s), 72°C for 10min; use M13 Primer M4 and Cel5A-3F2 as primers for the second round of PCR, the reaction conditions are: 94°C for 2min, 30 cycles (94°C for 30s, 52°C 30s, 72°C 90s), 72°C 10min. The two rounds of PCR products were analyzed by 1% agarose gel electrophoresis, the target band was recovered and ligated with pUCm-T (pUCm-T-cel5A3′), transformed into JM109, and then sent to Shanghai Sangon for sequencing.
Embodiment 2A
[0032] Cloning of embodiment 2Aus cel5A 5' end mRNA sequence
[0033] The first round of PCR amplification was performed using the Outer Primer and Cel5A-5R1 of the 5′-Full RACE Kit as primers. The reaction conditions were: 94°C for 3 minutes, 30 cycles (94°C for 30s, 55°C for 30s, 72°C for 60s), 10 min at 72°C; the second round of PCR was performed using Inner Primer and Cel5A-5R2 as primers, and the reaction conditions were: 3 min at 94°C, 30 cycles (30s at 94°C, 30s at 55°C, 60s at 72°C), and 10 min at 72°C. The two rounds of PCR products were analyzed by 1% agarose gel electrophoresis, the target band was recovered and ligated with pUCm-T (pUCm-T-cel5A5′), transformed into JM109, and then sent to Shanghai Sangon for sequencing.
Embodiment 3A
[0034] Cloning of Example 3 Aus cel5A 5' end promoter sequence
[0035] Using the processed A.usamii genomic DNA as a template, the first round of PCR used T-PrimerF and Cel5A-5R1 as primers, and the reaction conditions were: 94°C for 4min, 30 cycles (94°C for 30s, 55°C for 30s, 72°C for 60s ), 72°C for 10min; the second round of PCR uses T-PrimerF and Cel5A-5R2 as primers, and the reaction conditions are: 94°C, 4min, 30 cycles (94°C for 30s, 55°C for 30s, 72°C for 60s), 72°C for 10min . The two rounds of PCR products were analyzed by 1% agarose gel electrophoresis, the target band was recovered and ligated with pUCm-T (pUCm-T-cel5AP), transformed into JM109, and sent to Shanghai Sangon for sequencing after enzyme digestion and identification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com