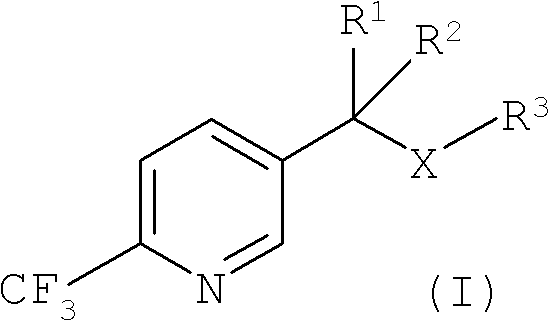
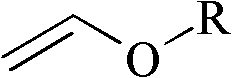
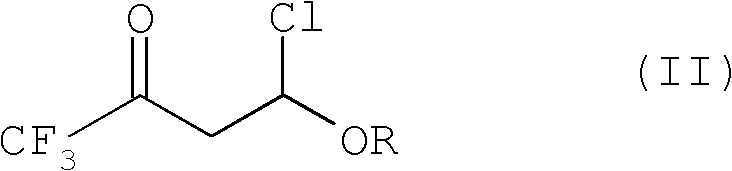
Improved process for the preparation of 2-trifluoromethyl-5-(1-substituted)alkylpyridines
A technology of alkylpyridine and trifluoromethyl, applied in the field of preparation of 2-trifluoromethyl-5-alkylpyridine, can solve the problems of expensive and unstable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Preparation of 5-(1-methylthio)ethyl-2-(trifluoromethyl)pyridine
[0089]
[0090] Step 1. Preparation of 1-(3-methylthiobut-1-enyl)pyrrolidine
[0091]
[0092] A dry 5000 milliliter (mL) round bottom flask equipped with a mechanical stirrer, nitrogen inlet, addition funnel, and thermometer was charged with 591 g (4.27 moles) of anhydrous potassium carbonate pellets and 1428 mL (17.1 moles) of anhydrous pyrrolidine. The mixture was stirred under an atmosphere of nitrogen and cooled to 4°C with an ice bath before 1050 mL (8.9 moles) of 3-methyl-thiobutyraldehyde was added at a rate to keep the temperature below 10°C. Once the addition was complete, the cooling bath was removed and the reaction mixture was allowed to reach room temperature. The reaction contents were then filtered through a sintered glass filter funnel to remove solids, which were washed with 200 mL of anhydrous diethyl ether. The filtrate was concentrated in vacuo on a rotary evaporat...
Embodiment 2
[0096] Example 2: Alternative preparation of 5-(1-methylthio)ethyl-2-(trifluoromethyl)pyridine
[0097]
[0098]Charge 240 mL of toluene into a 500 mL jacketed reactor equipped with a circulating bath (containing Syltherm 800), mechanical stirring, dry ice / acetone condenser, and thermowell (with digital monitoring), and then add 43.3 g (0.6 mol) ethyl vinyl ether (EVE). The reaction mixture was cooled to 0 °C, and then a 1 / 4" Teflon line was placed subsurface in the reaction mixture. Trifluoroacetyl chloride (TFAC) was bubbled through the Teflon line for 1 hour. Fluoroethylene resin tube until 95.3 g (0.72 moles) of trifluoroacetyl chloride reagent has been added. During the addition of TFAC, the internal reaction temperature rose from 2°C to 6°C. The circulating bath temperature was then set at 20°C, The reaction mixture was warmed to the circulating bath set point and stirred for an additional 20 minutes. At this point, GC analysis showed the presence of about 4% (GC rel...
Embodiment 3
[0103] Example 3: Alternative preparation of 5-(1-methylthio)ethyl-2-(trifluoromethyl)pyridine
[0104]
[0105] A 2-liter three-neck round bottom flask equipped with mechanical stirring, nitrogen blanket, and temperature probe was charged with 152.0 g (1.10 moles) of solid potassium carbonate, followed by ~1 liter of toluene. To this mixture was added 71.1 g (1.00 moles) of pyrrolidine. The reaction mixture was cooled in an ice-water bath, then 118.20 g (1.00 mole) of 3-methylthiobutanal was added continuously via an addition funnel over 1 hour and 16 minutes. The rate of aldehyde addition was adjusted to keep the internal reaction temperature below ~10°C. The ice-water bath was removed and the reaction mixture was allowed to warm to ambient temperature and stirred for an additional 3 hours 30 minutes. At this point, GC analysis indicated the presence of ~0.4% (relative area) of starting 3-methylthiobutyraldehyde. The reaction mixture was suction filtered, and the filte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com