Levo-gossypol for treating tumors or cyclodextrin clathrate compound of acetate thereof
A technology of inclusion compound of L-gossypol acetate and cyclodextrin, which is applied in the field of anti-tumor drug composition, and can solve problems such as unsatisfactory stabilization effect, low stabilization effect, and slow drug release.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
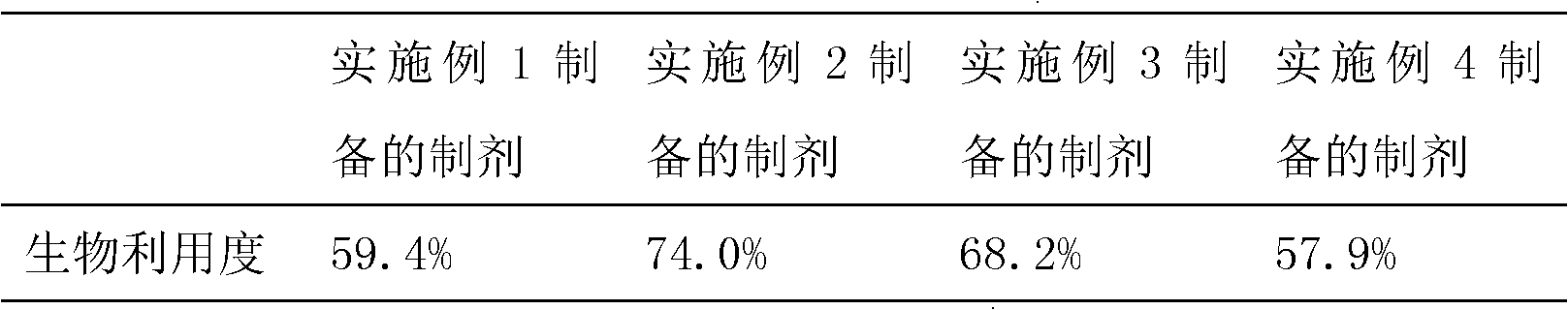
[0031] Embodiment 1 prepares the cyclodextrin inclusion compound of L-gossypol
[0032] The formula is:
[0033] L-Gossypol 5g
[0034] Macrogol 2000 3.5g
[0035] Hydroxypropyl-β-cyclodextrin (average degree of substitution 5.4) 40.5g
[0036] Proceed as follows:
[0037]1) Add hydroxypropyl-β-cyclodextrin, polyethylene glycol 2000 and L-gossypol to a small amount of absolute ethanol in a prescribed proportion, the amount of absolute ethanol per 1g of L-gossypol is 4ml, then add water to volume , prepared into a solution containing 1g L-gossypol or L-gossypol acetate per 140ml, magnetically stirred at 40°C for 5 hours, divided into vials, and half-tightened;
[0038] 2) freeze-drying:
[0039] a. Pre-freezing: quickly freeze the solution in the vials to -32°C, and keep freezing for 5 hours;
[0040] b. Primary drying: evacuate to 20Pa, then raise the temperature to -8°C at a rate of 8°C / hour, and dry at constant temperature for 6 hours;
[0041] c. Secondary drying: Af...
Embodiment 2
[0043] Embodiment 2 prepares the cyclodextrin inclusion complex of L-gossypol acetate
[0044] The formula is:
[0045] L-Gossypol Acetate 5g
[0046] Macrogol 1500 3.0g
[0047] Hydroxypropyl-β-cyclodextrin (average degree of substitution 5.7) 50g
[0048] Proceed as follows:
[0049] 1) Add hydroxypropyl-β-cyclodextrin, polyethylene glycol 1500 and L-gossypol acetate to a small amount of absolute ethanol in a prescribed proportion, and the amount of absolute ethanol per 1g of L-gossypol acetate is 3ml , then add water to constant volume, prepare a solution containing 1g of gossypol or gossypol acetate per 160ml, magnetically stir at 40°C for 5 hours, pack in vials, and half stopper;
[0050] 2) freeze-drying:
[0051] a. Pre-freezing: quickly freeze the solution in the vials to -40°C, and keep freezing for 5 hours;
[0052] b. Primary drying: evacuate to 40Pa, then raise the temperature to -12°C at a rate of 8°C / hour, and dry at constant temperature for 6 hours;
[00...
Embodiment 3
[0055] Example 3 Preparation of cyclodextrin inclusion compound of L-gossypol acetate
[0056] The formula is:
[0057] L-Gossypol Acetate 5g
[0058] Macrogol 2000 3.0g
[0059] Hydroxypropyl-β-cyclodextrin (average degree of substitution 5.5) 45g
[0060] Proceed as follows:
[0061] 1) Add hydroxypropyl-β-cyclodextrin, polyethylene glycol 2000 and gossypol acetate to a small amount of absolute ethanol in a prescribed proportion, and the amount of absolute ethanol per 1g of gossypol acetate is 4ml , then add water to constant volume, prepare a solution containing 1g L-gossypol or L-gossypol acetate per 180ml, magnetically stir at 40°C for 5 hours, pack in vials, and half stopper;
[0062] 2) freeze-drying:
[0063] a. Pre-freezing: quickly freeze the solution in the vials to -32°C, and keep freezing for 5 hours;
[0064] b. Primary drying: evacuate to 30Pa, then raise the temperature to -12°C at a rate of 8°C / hour, and dry at constant temperature for 6 hours;
[0065]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com