New method for preparing magnesium-aluminum hydrotalcite
A magnesium-aluminum hydrotalcite and new method technology, applied in chemical instruments and methods, aluminum compounds, inorganic chemistry and other directions, can solve the problems of high reaction cost, unfavorable environmental protection, difficult washing and filtration, and achieve mature technology, energy saving, The effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
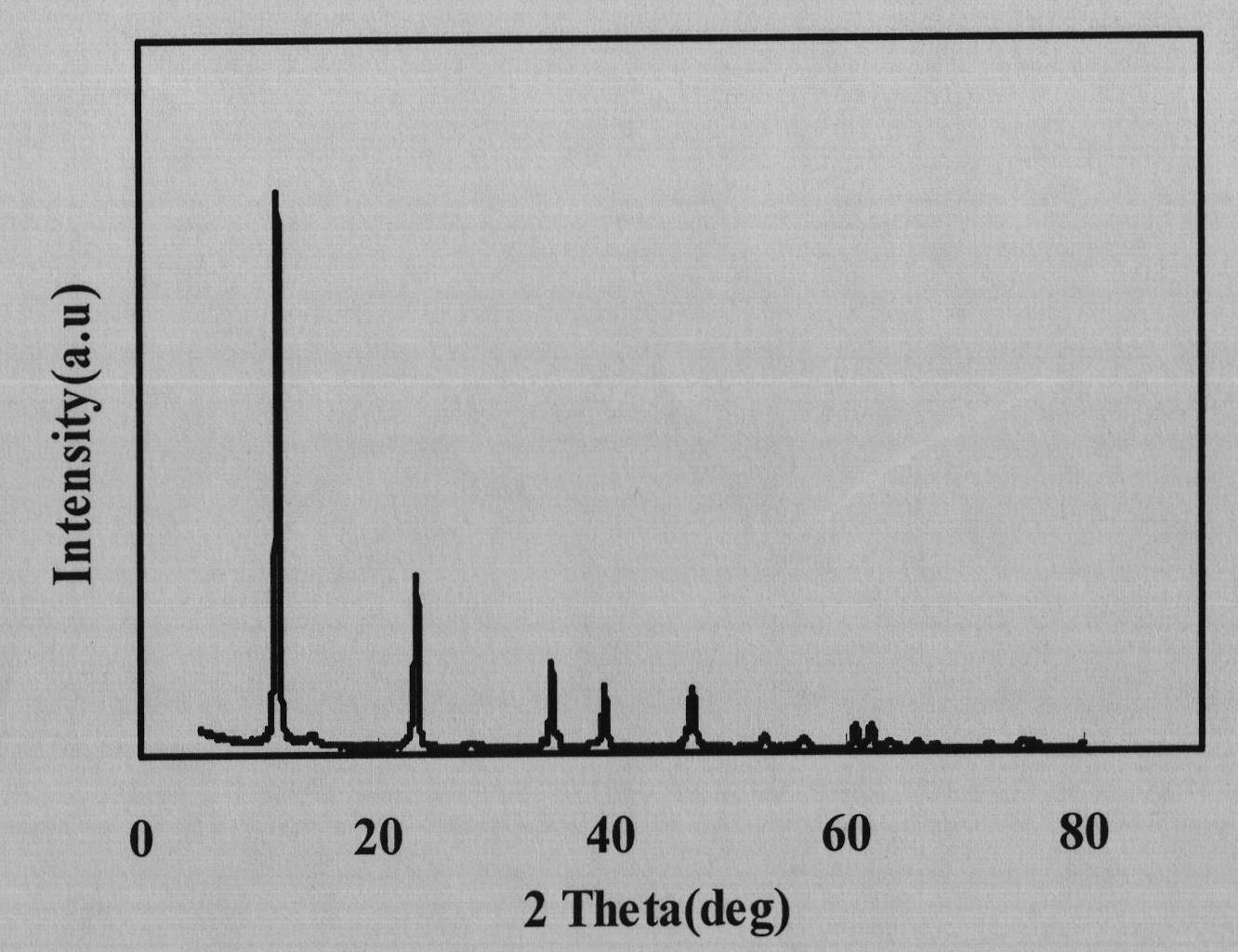
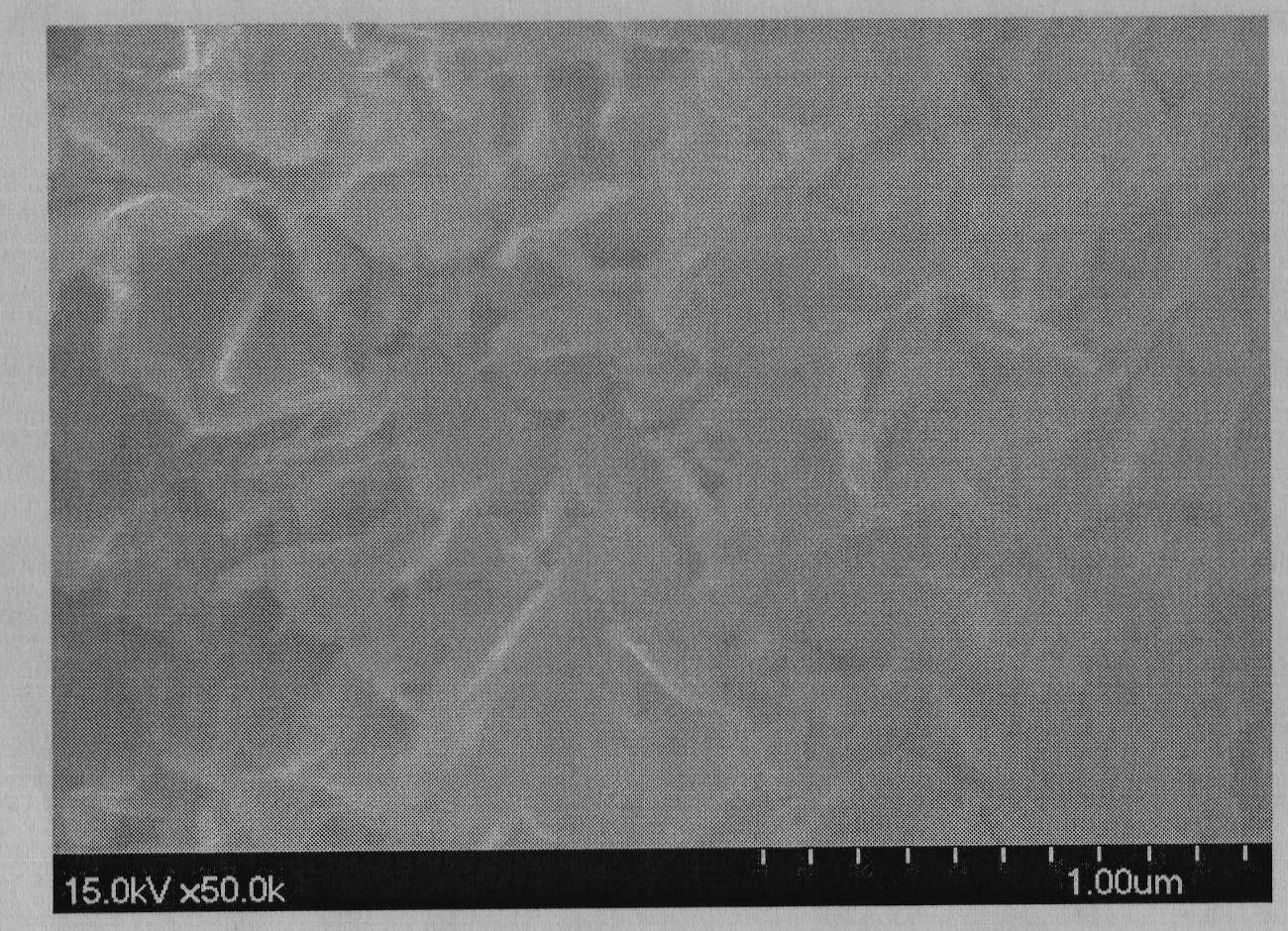
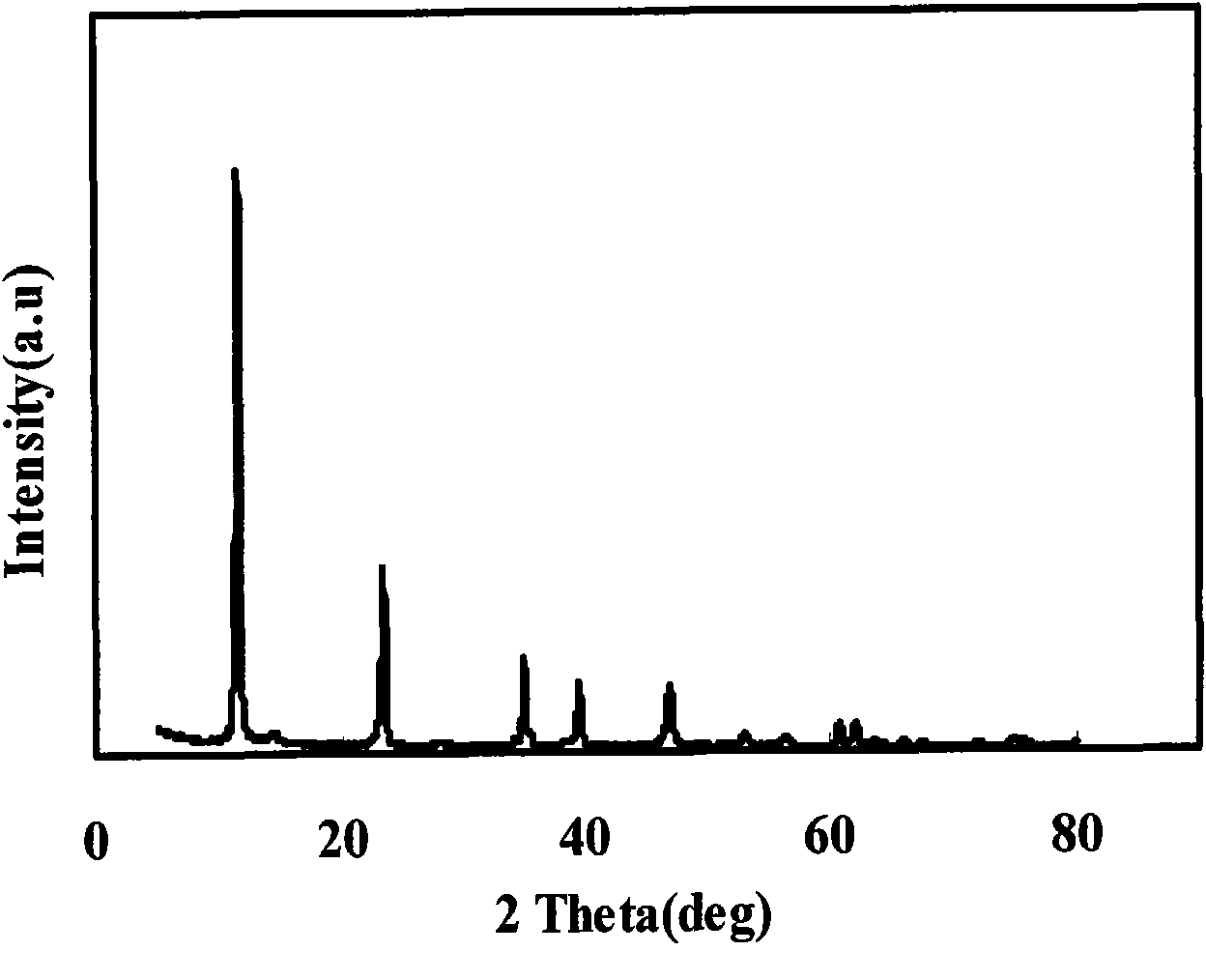
Image
Examples
Embodiment 1
[0028] Take a 500mL three-neck flask, add 160mL of 0.5mol / L aluminum chloride solution, add dropwise 120mL of 2mol / L ammonia water at 60°C, filter and wash twice after the addition, put the gelatinous aluminum hydroxide into 1L Take another 500mL three-neck flask, add 100mL of 2mol / L magnesium chloride solution, add 200mL of 2mol / L ammonia water dropwise at 60°C, filter and wash twice to obtain superfine magnesium hydroxide, and Magnesium was put into a 1L reaction kettle for later use; another 500mL three-neck flask was taken, and 20mL of 2mol / L magnesium chloride solution was added, and 40mL of 2mol / L ammonia water was added dropwise at 60°C. Carbon dioxide gas until the magnesium hydroxide is completely dissolved. Pour the clear solution into the above-mentioned 1L reaction kettle, cover and stir at 60°C for 2h, then add a surface modifier for modification, stir for 1h, and dry with a spray dryer.
Embodiment 2
[0030] Take a 500mL three-neck flask, add 160mL of 0.5mol / L aluminum nitrate solution, add 120mL of 2mol / L ammonia water dropwise at 70°C, filter and wash twice after the addition, put the gelatinous aluminum hydroxide into 1L of reaction Take another 500mL three-neck flask, add 100mL of 2mol / L magnesium chloride solution, add 200mL of 2mol / L ammonia water dropwise at 70°C, filter and wash twice to obtain superfine magnesium hydroxide, and Put it in a 1L reaction kettle for later use; take another 500mL three-neck flask, add 20mL of 2mol / L magnesium chloride solution, add 40mL of 2mol / L ammonia water dropwise at 70°C, filter and wash after the addition, add a small amount of water to stir, and pass through carbon dioxide Gas until the magnesium hydroxide is completely dissolved. Pour the clear solution into the above-mentioned 1L reaction kettle, cover and stir at 70°C for 2 hours, then add a surface modifier for modification, stir for 1 hour, and dry with a spray dryer.
Embodiment 3
[0032] Take a 500mL three-necked flask, add 160mL of 0.5mol / L aluminum chloride solution, add dropwise 120mL of 2mol / L ammonia water at 90°C, filter and wash twice after the addition, put the gelatinous aluminum hydroxide into 1L Take another 500mL three-neck flask, add 100mL of 2mol / L magnesium chloride solution, add 200mL of 2mol / L ammonia water dropwise at 90°C, filter and wash twice to obtain superfine magnesium hydroxide, and Put the magnesium into a 1L reaction kettle for later use; take another 500mL three-neck flask, add 20mL of 2mol / L magnesium chloride solution, add 2mol / L ammonia water 40mL dropwise at 90°C, filter and wash after the dropwise addition, add a small amount of water to stir, and pass Carbon dioxide gas until the magnesium hydroxide is completely dissolved. Pour the clear solution into the above-mentioned 1L reaction kettle, cover and stir at 90°C for 2 hours, then add a surface modifier for modification, stir for 1 hour, and dry with a spray dryer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com