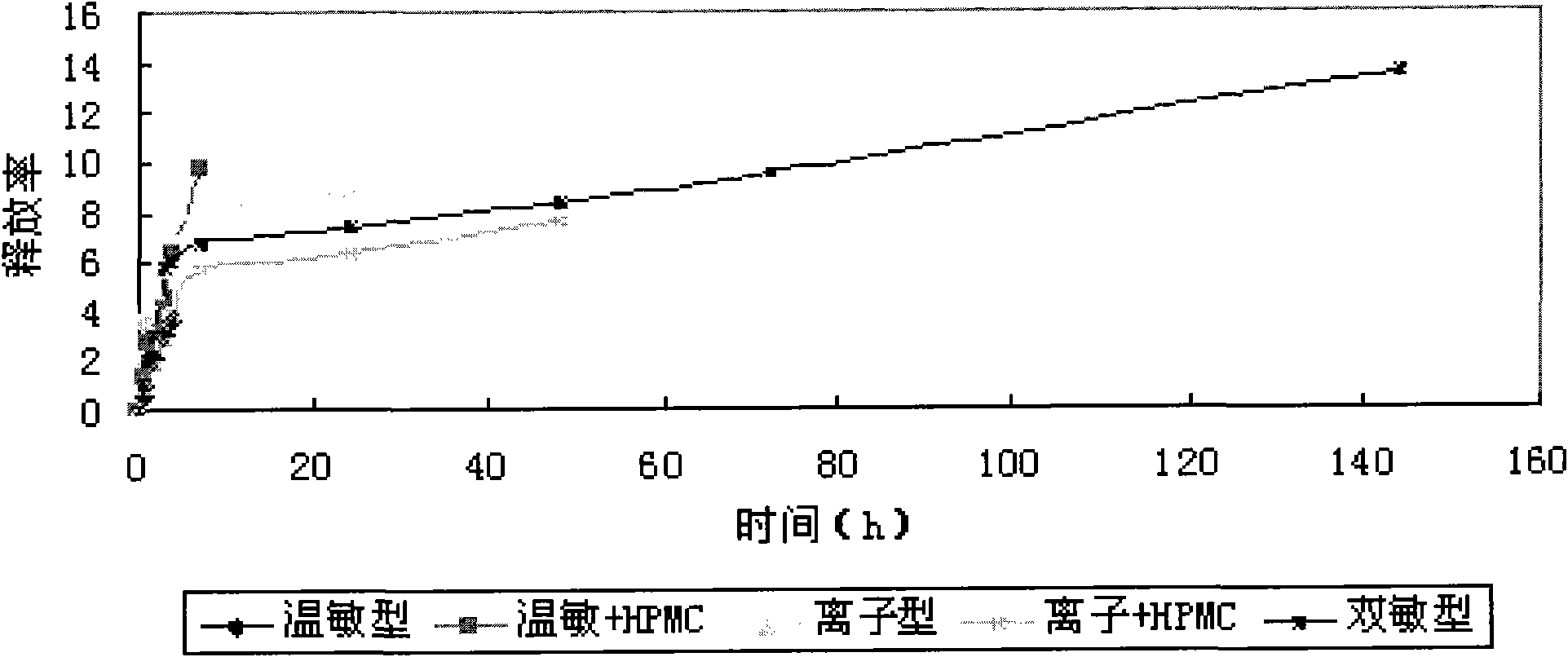
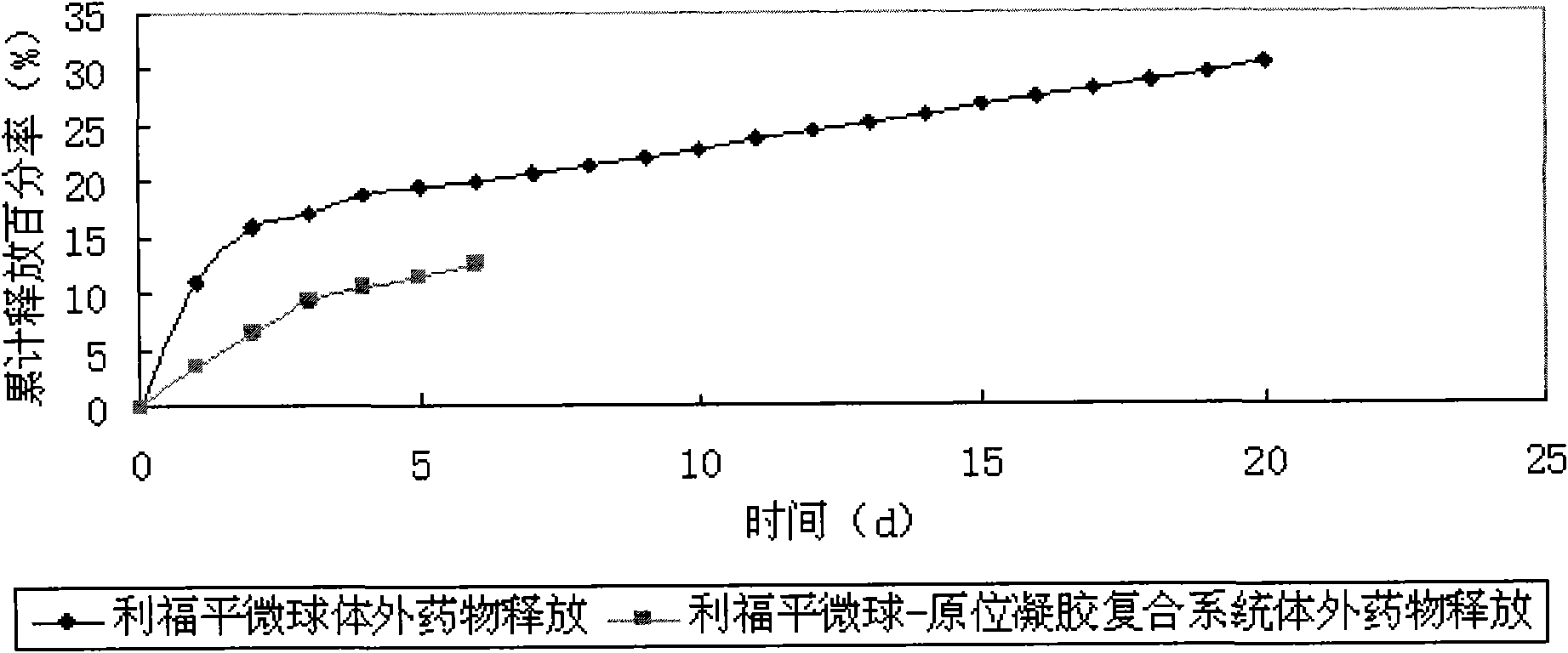
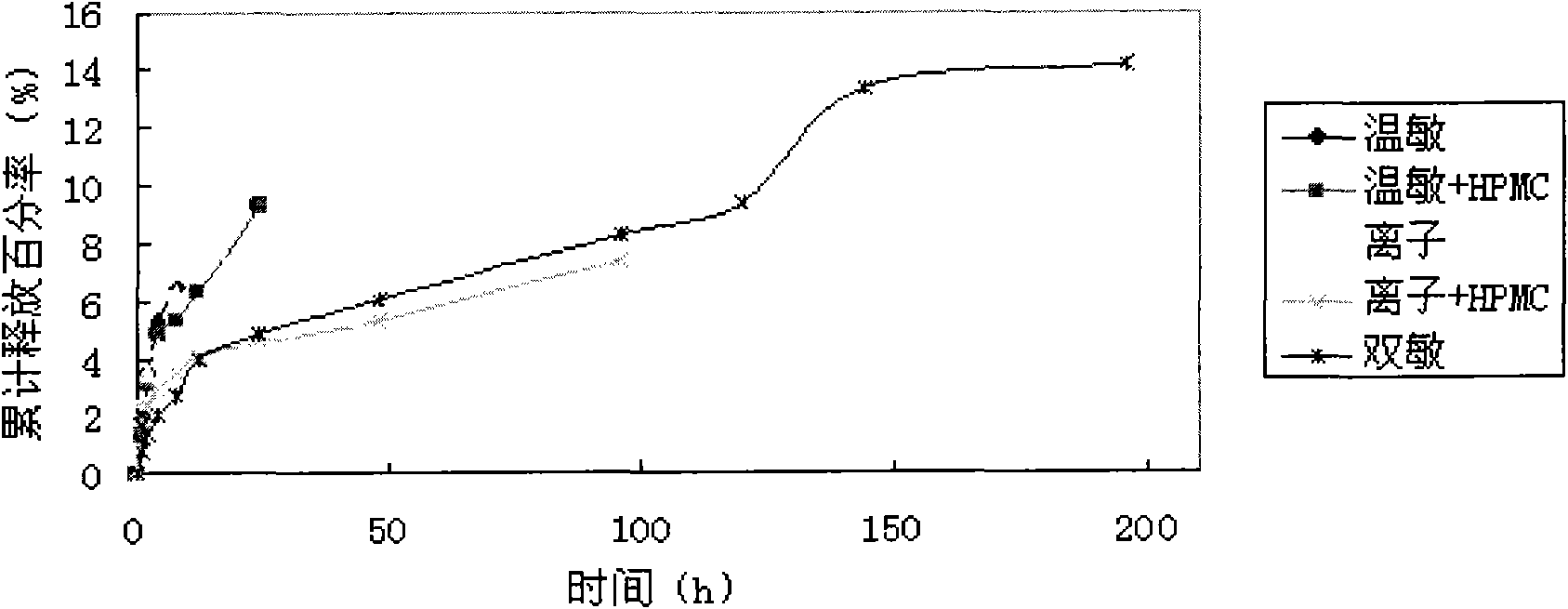
In-situ gel slow-release preparation for anti-tuberculosis drugs and preparation method thereof
A technology of in-situ gel and sustained-release preparations, applied in pharmaceutical formulations, antibacterial drugs, drug delivery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0448] Microsphere preparation of rifampicin:
[0449] Rifampicin (RFP) 15mg
[0450] Polylactic-co-glycolic acid (PLGA) 30mg
[0451] Polyvinyl alcohol (PVA) 0.5g
[0452] Dichloromethane 2ml
[0453] Deionized water 24ml
[0454] Preparation method of rifampicin microspheres: Weigh 30mg of PLGA and dissolve in dichloromethane, add 15mg of rifampicin, ultrasonically dissolve, as the organic phase; dissolve 0.5g of PVA in 24ml of deionized water, as the water phase. Under the condition of stirring at 1000rpm, slowly inject the organic phase into the water phase, stir at high speed for a certain period of time, stir at 600rpm until the organic phase is completely volatilized, collect the microspheres by suction filtration, wash with distilled water 3 times, and dry under reduced pressure to obtain the product.
[0455] The in situ gel preparation method is:
[0456] Poloxamer 407 25g
[0457] Poloxamer 188 3g
[0458] Sodium Alginate 2g
[0459] Hydroxypropyl methylcell...
Embodiment 2
[0464] Microsphere preparation of rifampicin:
[0465] Rifampicin (RFP) 19mg
[0466] Polylactic-co-glycolic acid (PLGA) 115mg
[0467] Polyvinyl alcohol (PVA) 1.7g
[0468] Dichloromethane 1ml
[0469] Deionized water 10ml
[0470] Preparation method of rifampicin microspheres: Weigh 115mg PLGA and dissolve in dichloromethane, add 19mg rifampicin, ultrasonically dissolve, as the organic phase; dissolve 1.7g PVA in 10ml deionized water, as the water phase. Under the stirring condition of 1200rpm, slowly inject the organic phase into the water phase, stir at high speed for a certain period of time, stir at 400rpm until the organic phase is completely volatilized, collect the microspheres by suction filtration, wash with distilled water 3 times, and dry under reduced pressure to obtain the product.
[0471] The in situ gel preparation method is:
[0472] Poloxamer 407 23.9g
[0473] Poloxamer 188 2g
[0474] Hydroxypropyl Methyl Cellulose 1.5g
[0475] Deionized water 10...
Embodiment 3
[0479] Microsphere preparation of rifampicin:
[0480] Rifampicin (RFP) 28mg
[0481] Polylactic-co-glycolic acid (PLGA) 40mg
[0482] Polyvinyl alcohol (PVA) 1.4g
[0483] Dichloromethane 1ml
[0484] Deionized water 21ml
[0485] Preparation method of rifampicin microspheres: Weigh 40mg PLGA and dissolve in dichloromethane, add 28mg rifampicin, ultrasonically dissolve, as the organic phase; dissolve 1.4g PVA in 21ml deionized water, as the water phase. Under the stirring condition of 1200rpm, slowly inject the organic phase into the water phase, stir at high speed for a certain period of time, stir at 400rpm until the organic phase is completely volatilized, collect the microspheres by suction filtration, wash with distilled water 3 times, and dry under reduced pressure to obtain the product.
[0486] The in situ gel preparation method is:
[0487] Sodium Alginate 1.3g
[0488] Hydroxypropyl methylcellulose 0.8g
[0489] Deionized water 100ml
[0490] Take the sodium a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com