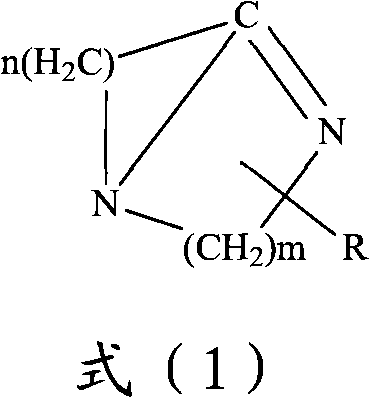
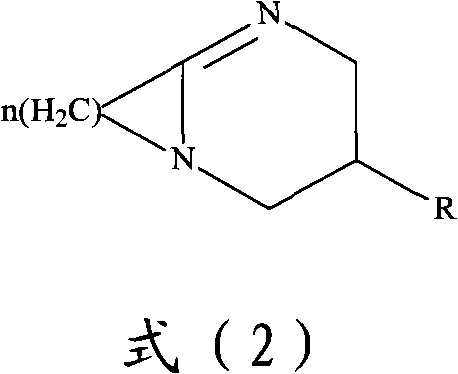
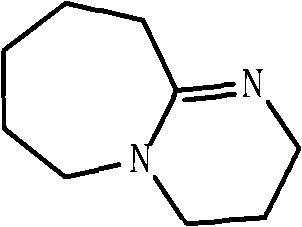
Method for preparing 2,2-dimethyl-3-hydroxy propanal
A technology of hydroxypropionaldehyde and dimethyl, which is applied in the field of preparation of chemical intermediates, can solve the problems of low conversion rate of raw materials, low product selectivity, short activity cycle, etc., and achieve easy recovery, mild reaction conditions, and simple post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 170g (2.12mol) of 37% aqueous formaldehyde solution in 1 liter of reactor, isobutyraldehyde 144g (2mol), N 2 Replace 3 times, add 2.80g (0.02mol) of DBU dropwise at room temperature, after the dropwise addition, the temperature of the reaction is raised to 35°C and continue to keep warm for 1h to obtain a colorless viscous liquid, evaporate unreacted isobutyraldehyde, add 50ml of deionized water, cooled to room temperature, a large amount of white solids precipitated, filtered to obtain 199.3g of 2,2-dimethyl-3-hydroxypropanal, melting point 89-90°C. 1 H NMR (400MHz, CDCl 3 , δ / ppm): 1.22 (s, 6H), 2.0 (s, 1H), 3.78 (s, 2H), 9.52 (s, 1H). The filtrate contains most of the DBU, which can be concentrated to remove most of the water and then recycled. The conversion rate of isobutyraldehyde is 99.3%, and the selectivity of hydroxypivalaldehyde is 98.4%.
Embodiment 2
[0033] Add 170g (2.12mol) of 37% aqueous formaldehyde solution in 1 liter of reactor, isobutyraldehyde 144g (2mo), N 2 Substitute 3 times, add 9-methyl-1,7-diazabicyclo[4,4,0]decene-62.80g (0.02mol) dropwise at room temperature, after the dropwise addition, the reaction temperature rises to 35°C and continues to keep warm After 1h, a colorless viscous liquid was obtained, unreacted isobutyraldehyde was evaporated, 50ml of deionized water was added, cooled to room temperature, a large amount of white solid was precipitated, filtered to obtain 198.5g of 2,2-dimethyl-3-hydroxypropane Aldehyde, melting point 89-90°C. 1 H NMR (400MHz, CDCl 3 , δ / ppm): 1.22 (s, 6H), 2.0 (s, 1H), 3.78 (s, 2H), 9.52 (s, 1H). The filtrate contains most of 9-methyl-1,7-diazabicyclo[4,4,0]decene-6, and can be recycled after being concentrated to remove most of the water. The conversion rate of isobutyraldehyde is 99.2%, and the selectivity of hydroxypivalaldehyde is 98.1%.
Embodiment 3
[0035] Add 170g (2.12mol) of 37% aqueous formaldehyde solution in 1 liter of reactor, isobutyraldehyde 144g (2mol), N 2 Substitute 3 times, add 10-methyl-1,8-diazabicyclo[5,4,0]undecene-73.08g (0.02mol) dropwise at room temperature, and the reaction temperature rises to 35°C after the dropwise addition Continue to keep warm for 1h to obtain a colorless viscous liquid, evaporate unreacted isobutyraldehyde, add 50ml deionized water, cool to room temperature, a large amount of white solid precipitates, filter to obtain 199.3g 2,2-dimethyl-3- Hydroxypropionaldehyde, melting point 89-90°C. 1 H NMR (400MHz, CDCl 3 , δ / ppm): 1.22 (s, 6H), 2.0 (s, 1H), 3.78 (s, 2H), 9.52 (s, 1H). The filtrate contains most of 10-methyl-1,8-diazabicyclo[5,4,0]undecene-7, and can be recycled after being concentrated to remove most of the water. The conversion rate of isobutyraldehyde is 99.5%, and the selectivity of hydroxypivalaldehyde is 98.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com