Preparation method of rebamipide intermediate
A technology of volume ratio and structural formula, applied in the preparation of 2-amino-3-[2(1 hydrogen)-quinolone-4-yl]propionate, the key intermediate field of rebamipide, which can solve the problem of easy-to-flush materials , incomplete reaction, low yield and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
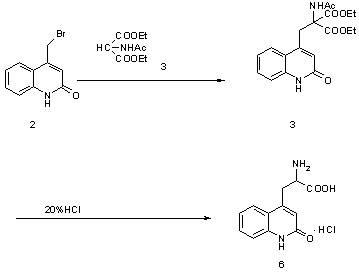
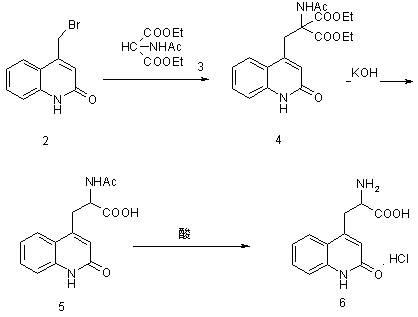
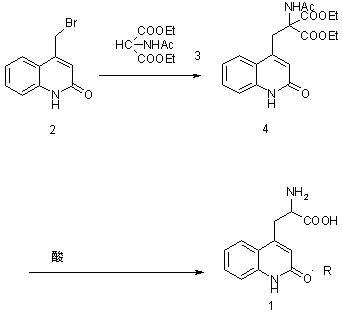
[0028] Example 1: Preparation of ethyl 2-acetylamino-2-carboxylate-3-(2-quinolinone-4 base)-propionic acid ethyl ester (compound of structural formula 4) in a 10000ml three-necked bottle, first put into 6000ml of water and ethanol, then 900 grams of 4-bromomethylbenzene-1 hydrogen-2-quinolinone (the compound of structural formula 2), 900 grams of diethyl acetamidomalonate (the compound of structural formula 3), 425 grams of sodium ethoxide gram, heated to 75°C, kept the temperature for 10 hours, adjusted the pH to 6 with acetic acid, added water equivalent to ethanol, cooled to 0°C to crystallize for 8 hours, filtered, and dried the filter cake under reduced pressure at 60°C for 8 hours to obtain 2 -Acetamido-2-formic acid ethyl ester-3-(2-quinolinone-4 base)-propionic acid ethyl ester (compound of structural formula 4) 1360 grams, yield 96.2%. The purity of ethyl 2-acetamido-2-carboxylate-3-(2-quinolinone-4yl)-propionate was determined by HPLC to be 98.3%.
Embodiment 2
[0029] Example 2: Preparation of 2-amino-3-[2(1hydrogen)-quinolone-4-yl]propion hydrochloride In a 1000ml three-necked flask, 280g of formic acid, 280g of concentrated hydrochloric acid, 2 -Acetamido-2-formic acid ethyl ester-3-(2-quinolinone-4 base)-propionic acid ethyl ester 250g, heat up to 85°C for 2 hours, then heat up to reflux, and keep the reaction under reflux for 24 hours , cooled to below 20°C, and filtered. The filter cake was dried at 60°C to obtain 163.6 g of 2-amino-3-[2(1hydrogen)-quinolon-4-yl]propionate hydrochloride with a yield of 91% and a purity of over 99.5% as determined by HPLC.
[0030] 1 H-NMR (DMSO- d6 , 400MHz): δ11.69(1H, HCl), δ8.55(3H, NH 2 , NH), δ7.81(1H, 8-Hor11-H), δ7.52(1H, 9-Hor10-H), δ7.36(1H, 8-Hor11-H ), δ7.22(1H, 9 -Hor10-H ), δ6.49(1H, 5-H ), δ4.14(1H, 2-H ), δ3.30-3.40(2H, 3-H ).
[0031] 13 C-NMR (DMSO- d6 , 400MHz): δ170(1C, 1-Cor6-C), δ161.36(1C, 1-Cor6-C), δ144.7(1C, 7-C), δ139.29(1C, 12-C), δ130.54(1C, 8-Cor9-Cor10-Cor11...
Embodiment 3
[0033] Embodiment 3: Preparation of 2-amino-3-[2(1 hydrogen)-quinolone-4-yl] propionate hydrochloride In a 1000ml three-necked flask, drop into 280g of acetic acid, 280g of concentrated hydrochloric acid, 2 prepared in Example 1 -Acetamido-2-formic acid ethyl ester-3-(2-quinolinone-4 base)-propionic acid ethyl ester 250g, heat up to 87°C for 2 hours, then heat up to reflux, and keep the temperature under reflux for 24 hours , cooled to below 20°C, and filtered. The filter cake was dried at 60°C to obtain 172.6 g of 2-amino-3-[2(1hydrogen)-quinolon-4-yl]propionate hydrochloride with a yield of 96% and a purity of over 99.5% as determined by HPLC.
[0034] 1 H-NMR (DMSO- d6 , 400MHz): δ11.72(1H, HCl), δ8.69(3H, NH 2 , NH), δ7.83(1H, 8-Hor11-H), δ7.48(1H, 9-Hor10-H), δ7.30(1H, 8-Hor11-H ), δ7.19(1H, 9 -Hor10-H ), δ6.52(1H, 5-H ), δ4.10(1H, 2-H ), δ3.25-3.34(2H, 3-H ).
[0035] ESI-MS: m / z267[M-H] + , m / z231[M-HCl-H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com