Method for preparing gemcitabine hydrochloride and intermediate thereof with high selectivity
A gemcitabine hydrochloride, high selectivity technology, applied in the field of highly selective preparation of gemcitabine hydrochloride and its intermediates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
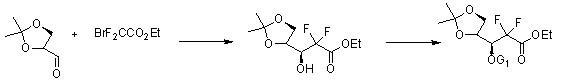
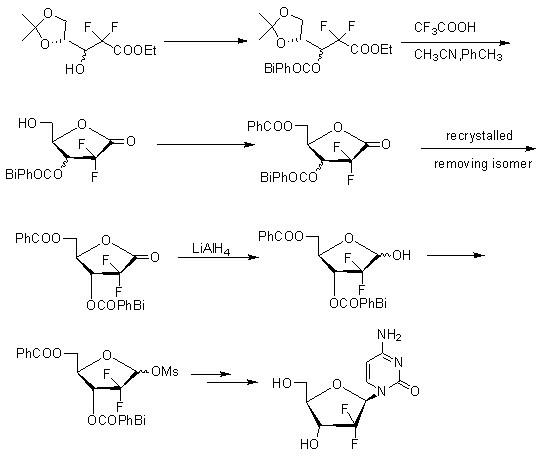
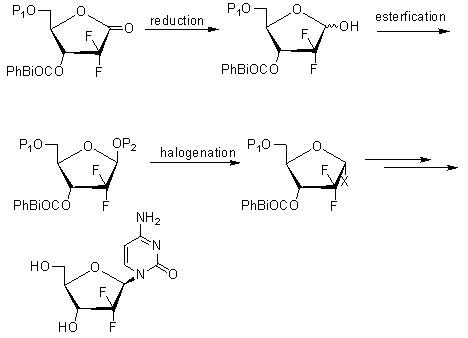
[0054] A highly selective method for preparing gemcitabine hydrochloride and its intermediates, the process is:
[0055]
[0056] 2 3 4
[0057]
[0058] 5 6 7
[0059]
[0060]
[0061] 10 1
[0062] Wherein the synthesis of compound (3):
[0063] Add 250 milliliters of tetrahydrofuran, 130 grams of zinc powder (1.98mol), and 5.1 milliliters of dibromoethane into the reaction flask, stir, heat up to 40°C, add 7.6 milliliters of trimethylchlorosilane, continue stirring at this temperature for 30 minutes, and then Raise to 60°C, slowly add dropwise 400 ml of tetrahydrofuran solution dissolved with 308 g of D-glyceraldehyde acetonide (compound 2) (2.36 mol) and 255 ml (1.98 mol) of ethyl bromodifluoroacetate, and heat up to reflux for reaction 2 hours, then cooled to room temperature; the reaction solution was poured into a mixed solution of 3000 grams of ice, 800 milliliters of ether and 2500 milliliters of 1M hydrochloric acid, and stirred for 30 minutes. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com