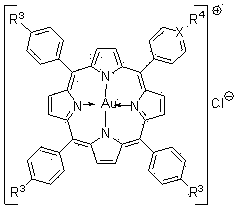
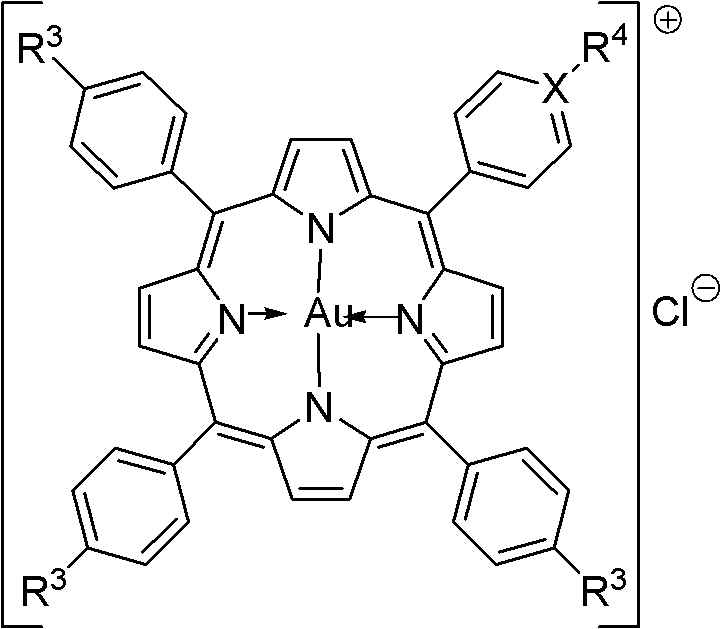
Water-soluble porphyrin gold (III) anticancer compounds and preparation method thereof
A porphyrin gold, water-soluble technology, applied in the field of water-soluble porphyrin gold anti-cancer compounds and its preparation, can solve the problems of being insoluble in water, etc., and achieve the effect of enhancing water solubility, good anti-cancer activity, and strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Chloride 5-(N-methyl-4-pyridyl iodide)-10,15,20-triphenylporphyrin gold (G2) compound and its preparation method.
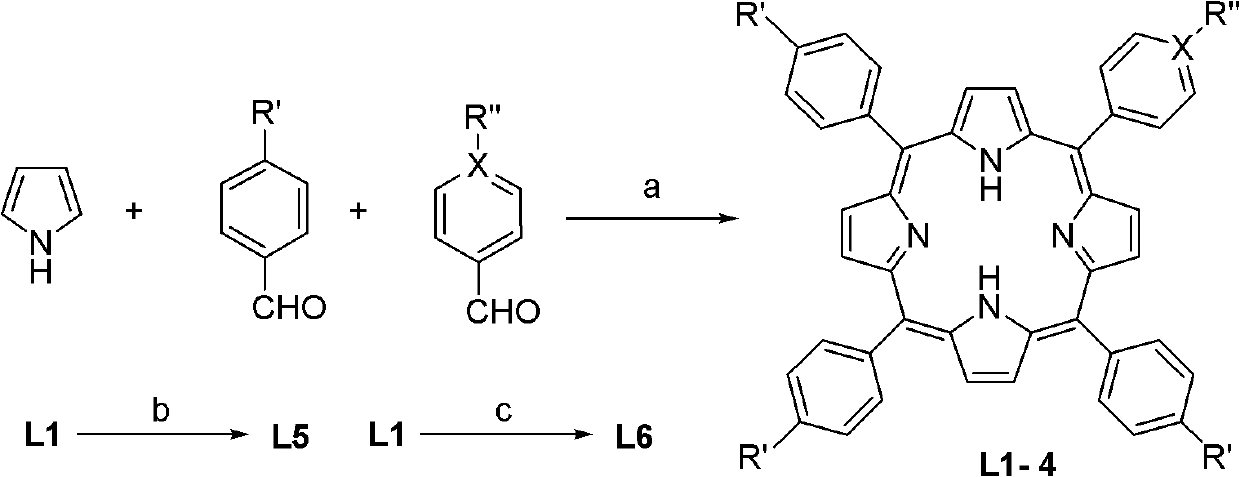
[0031] Add 200 mL of propionic acid, benzaldehyde (60 mmol) and 4-pyridine aldehyde (20 mmol) into a 250 mL round bottom flask, and heat to reflux. After mixing 5.53mL (80mmol) freshly distilled pyrrole and 10mL propionic acid, slowly add it dropwise to the propionic acid solution within 30min. Then continue to react for 1 h, cool to room temperature, precipitate solid, filter, and wash the solid with methanol and hot water at 50-80°C, and dry. 5-(4-pyridyl)-10,15,20-triphenylporphyrin (L2) was obtained with a yield of 6.5%: mp>250°C; 1 H NMR (CDCl 3 , 600MHz), δ (ppm): -2.82 (s, 2H, inner-NH), 7.75-7.80 (m, 9H, Ph-CH), 8.17 (d, J=5.4Hz, 2H, Py-CH), 8.21(d, J=6.6Hz, 6H, Ph-CH), 8.79(d, J=4.2Hz, 2H, Py-CH), 8.86-8.90(m, 6H, Por-CH), 9.03(s, 2H , Por-CH); IR(KBr): υ3446(s), 1634(w), 1590(w), 1473(w), 1396(w), 1351(w), 1070(w), 970(w) , 798(m),...
Embodiment 2
[0034] Example 2 Chlorination of 5,10,15,20-tetrakis(4-sodium carboxylate) phenylporphyrin gold (G3) compound and its preparation method.
[0035] Add 200mL propionic acid and 4-methoxylbenzaldehyde (80mmol) into a 250mL round bottom flask, and heat to reflux. After mixing 5.53mL (80mmol) freshly distilled pyrrole and 10mL propionic acid, slowly add it dropwise to the propionic acid solution within 30min. Then continue to react for 1 h, cool to room temperature, precipitate solid, filter, and wash the solid with methanol and hot water at 50-80°C, and dry. 5,10,15,20-tetrakis(4-methoxyacyl)phenylporphyrin (L3) was obtained with a yield of 22.0%: mp>250°C; 1 H NMR (600MHz, CDCl 3 ), δ (ppm): -2.79 (s, 2H, inner-NH), 4.11 (s, 12H, -COOCH 3 ), 8.29 (d, J=7.8Hz, 8H, Ph-CH), 8.44 (d, J=7.8Hz, 8H, Ph-CH), 8.81 (s, 8H, Por-CH); IR(KBr): υ3425(m), 2919(w), 1724(s), 1607(w), 1435(w), 1383(w), 1277(m), 1108(m), 965(w), 803(w), 762(w)em -1 ; UV-Vis (CH 2 Cl 2 ) / nm 417, 515, 549, 5...
Embodiment 3
[0038] Example 3 Chloride 5-(4-sodium carboxylate)phenyl-10,15,20-triphenylporphyrin gold (G4) compound and its preparation method.
[0039] Add 200 mL of propionic acid, benzaldehyde (60 mmol) and 4-methoxybenzaldehyde (20 mmol) into a 250 mL round bottom flask, and heat to reflux. After mixing 5.53mL (80mmol) freshly distilled pyrrole and 10mL propionic acid, slowly add it dropwise to the propionic acid solution within 30min. Then continue to react for 1 h, cool to room temperature, precipitate solid, filter, and wash the solid with methanol and hot water at 50-80°C, and dry. 5-(4-methoxyacyl)phenyl-10,15,20-triphenylporphyrin (L4) was obtained in a yield of 9.0%: mp>250°C; 1 H NMR (CDCl 3 , 600MHz), δ (ppm): -2.79 (s, 2H, inner-NH), 4.11 (s, 3H, -COOCH 3 ), 7.74-7.79 (m, 9H, Ph-CH), 8.21 (d, J=6.6Hz, 6H, Ph-CH), 8.31 (d, J=7.8Hz, 2H, Ph-CH), 8.44 (d , J=7.8Hz, 2H, Ph-CH), 8.79(d, J=4.2Hz, 2H, Por-CH), 8.85(s, 6H, Por-CH); IR(KBr): υ3442(s), 3319(w), 2924(w), 2853(w), 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com