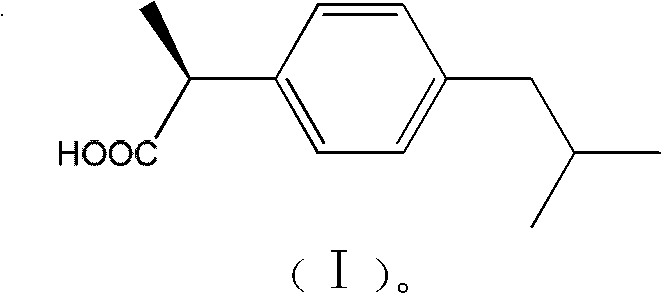
Method for splitting ibuprofen
A technology for the resolution of ibuprofen methyl ester, which is applied in the field of resolution of the compound ibuprofen [2-propionic acid], can solve the problem that the optical purity of d-ibuprofen is difficult to reach the ideal level, the operation is cumbersome, and the yield Low efficiency and other problems, to achieve the effect of reducing the pollution of "three wastes", high reaction yield and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 S (+)- ibuprofen methyl ester
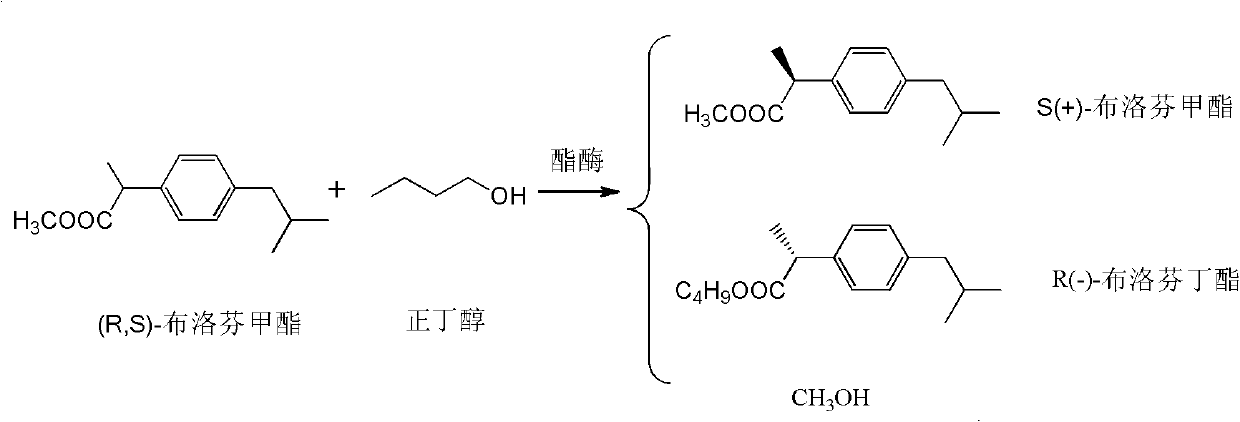
[0035] Dissolve 22g (100mmol) of racemic ibuprofen methyl ester and 5.9g (80mmol) of n-butanol in 400mL of dioxane, then add 10g of Novozym 435, and shake at 39°C for 20h. After the reaction, the immobilized enzyme was filtered off. The filtrate is distilled and concentrated to 30 to 50mL, separated on a silica gel column (eluent is isopropyl ether) to obtain about 10g of S(+)-ibuprofen methyl ester, and the yield is about 91% (based on S(+)-buprofen profen methyl ester).
Embodiment 2
[0036] The preparation of embodiment 2 S (+)- ibuprofen methyl ester
[0037] Dissolve 22g (100mmol) of racemic ibuprofen methyl ester and 5.9g (80mmol) of n-butanol in 400mL of dioxane, then add 10g of Novozym 435 (recovered from Example 1), and shake at 39°C for 22h . After the reaction, the immobilized enzyme was filtered off. The filtrate is distilled and concentrated to 30 to 50mL, separated on a silica gel column (eluent is isopropyl ether) to obtain about 10g of S(+)-ibuprofen methyl ester, and the yield is about 91% (based on S(+)-buprofen profen methyl ester).
Embodiment 3
[0039] Dissolve 22g (100mmol) of racemic ibuprofen methyl ester and 59g (800mmol) of n-butanol in a mixture of 500mL of methyl tert-butyl ether and petroleum ether (1:1 by volume), then add 10g of Novozym 435 , Shake the reaction at 0°C for 72h. After the reaction, the immobilized enzyme was filtered off. The filtrate is distilled and concentrated to 30 to 50ml, separated on a silica gel column (eluent is isopropyl ether) to obtain about 8g of S(+)-ibuprofen methyl ester, and the yield is about 92.7% (based on S(+)-buprofen profen methyl ester).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com