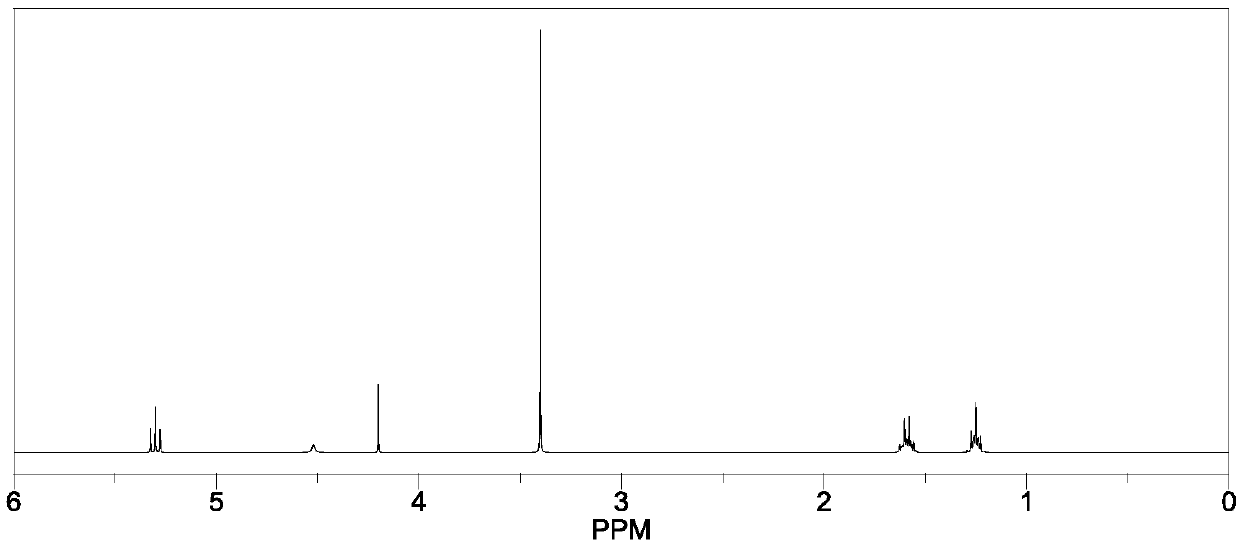
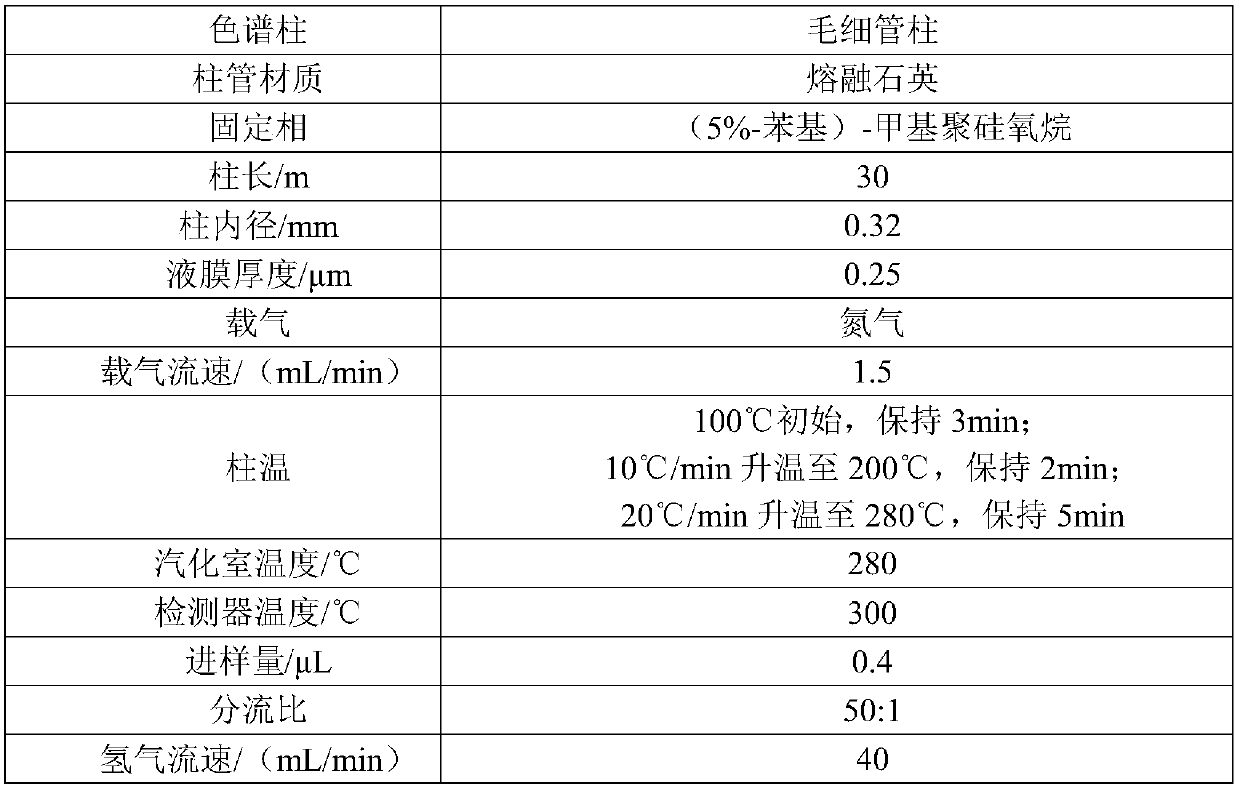
Preparation method of dialdehyde hemiacetal
A technology of hemiacetal and dialdehyde, which is applied in the field of preparation of dialdehyde hemiacetal, achieves the effects of simple operation, reducing the generation of by-products, and improving anti-oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Preparation of loaded catalyst: dissolving rhodium chloride in the hydrochloric acid aqueous solution of 1mol / L to obtain concentration is the active component solution (in terms of rhodium element) of 5wt%, and palladium chloride is dissolved in the hydrochloric acid aqueous solution of 1mol / L to obtain concentration 5wt% active component solution (calculated as Pd element), then impregnate 10kg of silica carrier particles (16 mesh, specific surface area 300m 3 / g), the immersion time was 4h, and then dried at 110°C for 24h to obtain a solid; then according to the amount in Table 2, the solid was impregnated with 5wt% ammonium dihydrogen phosphate aqueous solution for 3h, then dried at 110°C for 24h, and then dried at 550 Calcined at ℃ for 4 hours to obtain a supported catalyst, which is recorded by HP number, and the preparation formula and composition of the catalyst are shown in Table 2.
[0066] Table 2 Catalyst preparation formula and composition
[0067]
[0...
Embodiment 2
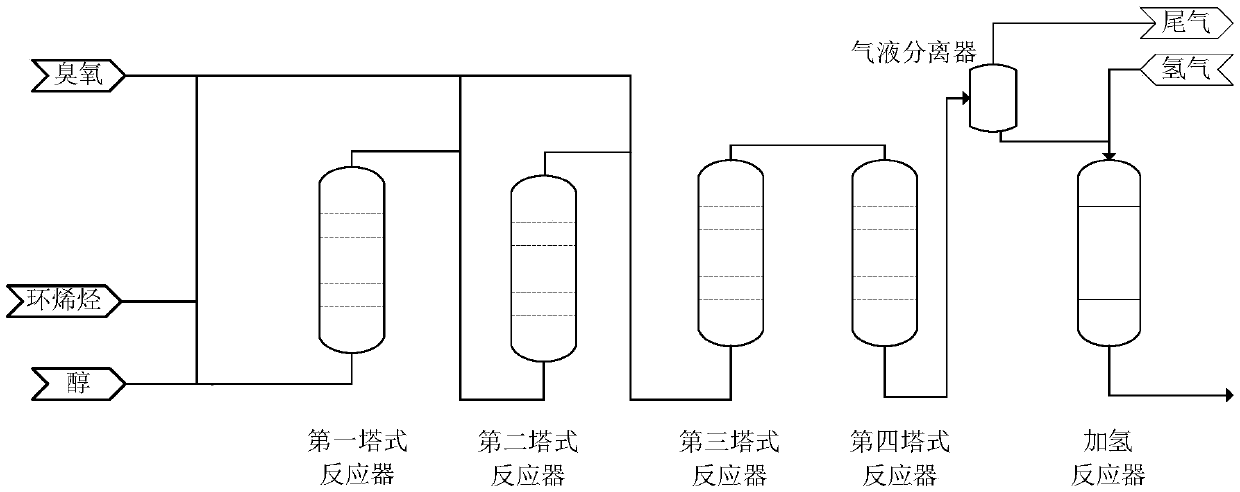
[0070] use figure 1 The process flow diagram shown in the reaction.
[0071] Oxidation reaction: Set the flow rate of cyclopentene to 1.5kg / h and the flow rate of methanol to 8.5kg / h. After mixing the two, add them to a tower reactor. After all the tower reactors are full of liquid, control the temperature in the reactor to At 15°C, ozone was gradually introduced into the first, second, and third tower reactors respectively, and the total flow rate of ozone feed was 1109.6g / h, among which, the feed flow rate containing ozone in the first stage tower reactor accounted for the total flow rate 50%, the feed of the second-stage tower reactor contains 40% of the ozone flow, the feed of the three-stage tower reactor contains 10% of the ozone flow, and finally the reaction liquid flowing out from the third tower reactor passes through the fourth tower reaction The device is aged, the system pressure is controlled to 0.5MPa, the total residence time of the reaction is 4h, and the oxi...
Embodiment 3
[0074] Use different catalysts to carry out hydrogenation reaction, all the other techniques and parameters are identical with embodiment 2. The results are shown in Table 3.
[0075] Table 3 Reaction performance of different catalysts
[0076]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com