Process for making cyclohexylbenzene
A kind of technology of cyclohexylbenzene and dicyclohexylbenzene, applied in the field of preparation of cyclohexylbenzene, can solve problems such as difficult separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] U.S. Patent Application No. 61 / 047,821, filed April 25, 2008, the disclosure of which is incorporated by reference in its entirety, discloses a process for the preparation of cyclohexylbenzene in which benzene and hydrogen are reacted with a first catalyst in an atmosphere sufficient to produce ring-containing The first effluent stream of hexylbenzene, cyclohexane and unreacted benzene is contacted under hydroalkylation conditions. Then at least a portion of the first effluent stream containing cyclohexane and unreacted benzene is contacted with a second catalyst under dehydrogenation conditions to convert at least a portion of the cyclohexane to benzene and / or biphenyl, and A second effluent stream containing less cyclohexane than the first effluent stream is produced. At least a portion of the second effluent stream is recycled back to the first contacting (hydroalkylation) step.
[0022] In the process of the present invention, cyclohexylbenzene is produced by conta...
Embodiment 1-9
[0061] Examples 1-9. CHB production under various process conditions
[0062] 8 grams of the catalyst prepared above were charged into a stainless steel fixed bed microreactor. The reactor had a 1 / 2-inch inner diameter with a 1 / 8-inch thermowell in the center of the entire catalyst bed. The catalyst was pretreated with 100 cubic centimeters per minute (cc / min) of hydrogen at 300°C and 1 atmosphere (atm) for 2 hours (hr). After cooling to 155°C in hydrogen, benzene was fed to the reactor via a syringe pump at 60 cc / hour for 1 hr while the reactor pressure was increased to 150 pounds per square inch gauge (psig). The benzene rate was then reduced to 0.52 weight hourly space velocity (WHSV) and the hydrogen / benzene molar ratio was adjusted to 1.28. The liquid product was collected in cold product hydrazine and analyzed off-line. Catalyst performance was evaluated using various test conditions by varying four process variables. Table 1 shows these process variables and their r...
Embodiment 10 and 11
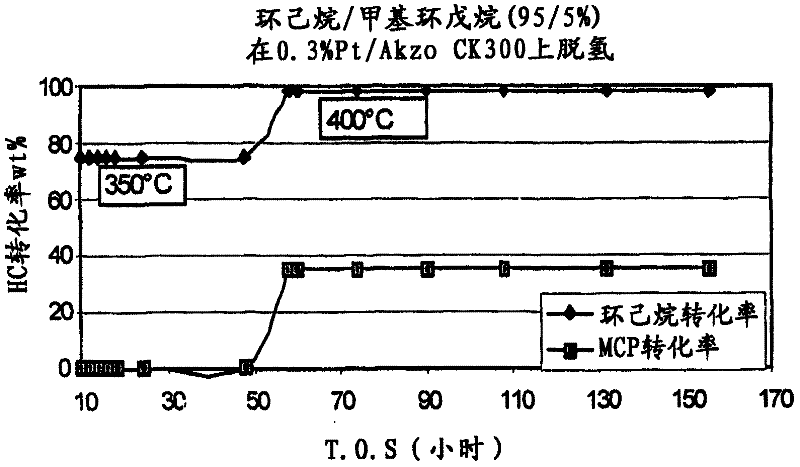
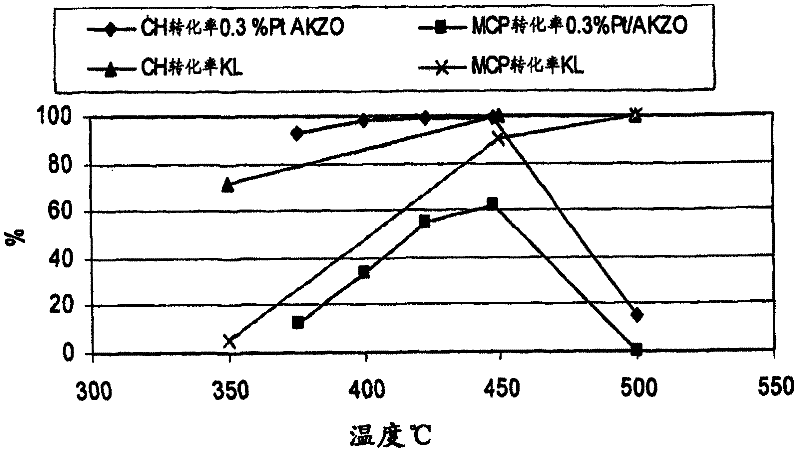
[0066] Examples 10 and 11: Cyclohexane / methylcyclopentane dehydrogenation
[0067] The reactor used in the experiments was stainless steel with dimensions 22 inches (in.) long x 3 / 8 in. outside diameter (o.d.) x 0.035 in. wall thickness. A piece of 8 3 / 4 in. long x 1 / 4 in.o.d stainless steel tubing was used at the bottom of the reactor as a spacer and to support the catalyst in the isothermal zone of the furnace. A 1 / 4 in. glass wool plug was placed on top of the spacer to keep the catalyst in place. A 1 / 8 in. thermowell was placed in the catalyst bed, long enough to accommodate a temperature sweep across the catalyst bed.
[0068] The catalyst was pressed into pellets, then crushed and sieved to 20-40 US mesh. Typically, 2.5 grams (g) of catalyst pre-screened to 20-40 mesh was diluted to a volume of 5.5 cc with quartz chips of the same size. The mixed portion of catalyst is then loaded into the reactor from the top. The catalyst bed is typically 12.5 cm. long. A 1 / 4 in. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com