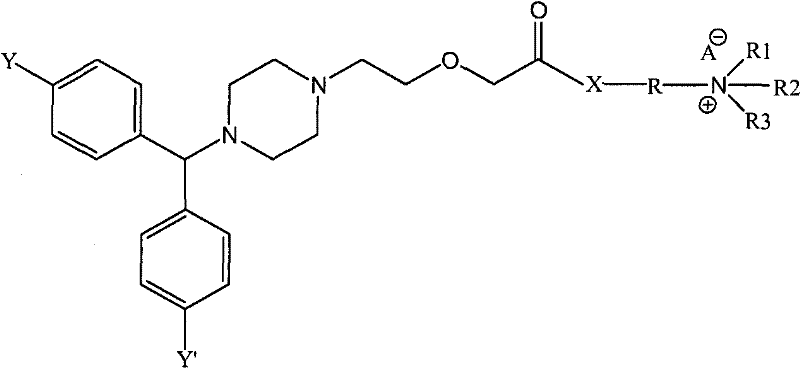
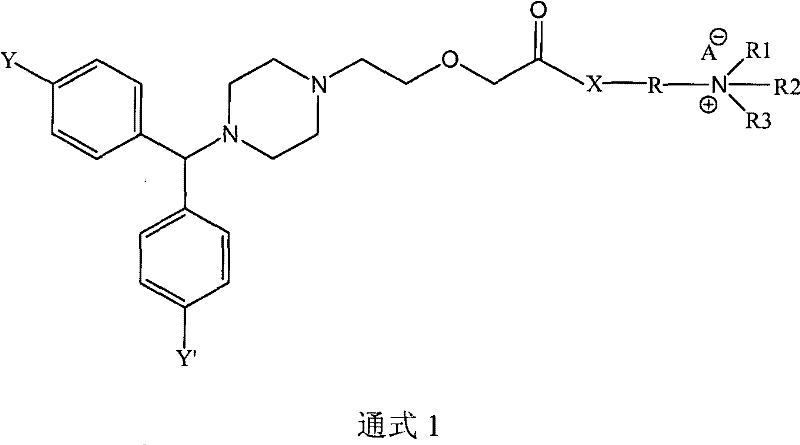
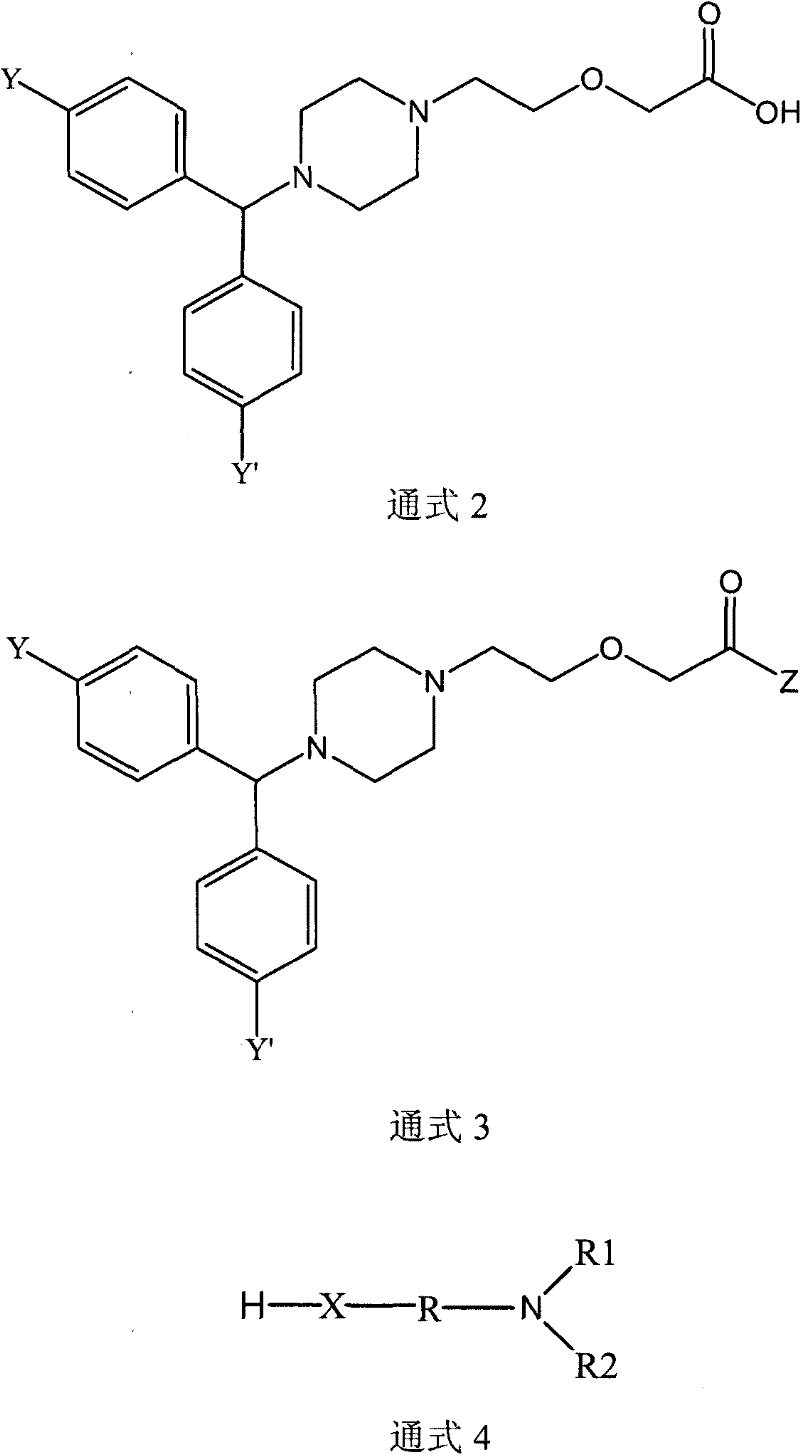
Cetirizine derivatives, and composition and application thereof
A composition and compound technology, which is applied in the direction of drug combination, respiratory system diseases, skin diseases, etc., can solve the problems of low transdermal efficiency, difficult to achieve effective blood drug concentration, and no guarantee of therapeutic effect, and achieve the effect of improving efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] The preparation of embodiment 1 compound
[0084](1) Preparation of 2-[2-[4-[(4-chlorophenyl)benzyl]-1-piperazinyl]ethoxy]diethylaminoethyl acetate
[0085] 38.89 g of cetirizine was dissolved in 100 ml of chloroform, and the mixture was cooled to 0°C. 20.63 g of 1,3-dicyclohexylcarbodiimide (DCC) was added to the reaction mixture, and stirred at 0° C. for 30 minutes. 11.72 g of 2-diethylaminoethanol was added to the reaction mixture, and stirred at room temperature for 3 hours. 6.01 g of acetic acid was added to the reaction mixture with stirring. The solid by-product was removed by filtration, washed three times with 50 ml of chloroform, and the organic phase was evaporated. After drying, 50.04 g of the hygroscopic target product were obtained. 1 H-NMR (400MHz, CDCL 3 ): δ: 1.56(t, 6H), 2.20(s, 3H), 2.51(t, 2H), 2.71(s, 8H), 3.28(q, 4H), 3.52(t, 2H), 3.60(t, 2H), 4.33(s, 2H), 4.52(t, 2H), 5.14(s, 1H), 6.95(s, 1H), 7.17(s, 2H), 7.26(s, 1H), 7.35(m, 6H ).
[008...
Embodiment 2
[0096] Embodiment 2 tests in vivo and measures transdermal rate
[0097] 1. Preparation of test solution
[0098] Need test solution 1: be dissolved in the 10% (W / V) cetirizine suspension of 1ml isopropanol;
[0099] Test solution 2: 10% (W / V) 2-[2-[4-[(4-chlorophenyl)benzyl]-1-piperazinyl]ethoxy in 1ml of isopropanol ] diethylaminoethyl acetate solution;
[0100] Test solution 3: 10% (W / V) 2-[2-[4-[(4-chlorophenyl)benzyl]-1-piperazinyl]ethoxy in 1ml of isopropanol ] dimethylaminoethyl acetate solution;
[0101] Need test solution 4: be dissolved in the levocetirizine suspension of 10% (W / V) of 1ml isopropanol;
[0102] Test solution 5: 10% (W / V) (R)-2-[2-[4-[(4-chlorophenyl)benzyl]-1-piperazinyl dissolved in 1ml isopropanol ]ethoxyl]acetate diethylaminoethyl acetate solution;
[0103] Test solution 6: 10% (W / V) (R)-2-[2-[4-[(4-chlorophenyl)benzyl]-1-piperazinyl dissolved in 1ml isopropanol ] Ethoxyl] diethylaminoethylthioacetate hydrochloride solution;
[0104] Test s...
Embodiment 3
[0115] The mensuration of embodiment 3 antihistamine efficacy
[0116] (1) Effects on rat skin vascular permeability
[0117]Get 80 white SD rats, divide them into 10 groups at random, cut their hair at 1.5cm on both sides of the back midline of the rats, 2 points on each side, with an interval of 2cm, and administer 5mg / kg (body weight) cetirizine percutaneously the next day , levocetirizine, eflurizine (normal saline suspension containing 10% ethanol), 6 kinds of compounds of general formula 1 prepared in Example 1 (normal saline solution containing 10% ethanol), and 10ml / kg (body weight) normal saline containing 10% ethanol. One hour after the administration, intradermally inject 0.1ml of histamine phosphate saline solution (containing 50 μg of histamine), inject 2 points, and simultaneously inject 1% (W / V) Evans blue normal saline solution 1ml / kg into the tail vein ( Body weight), the animals were killed after 30 minutes, the blue-stained skin was removed and cut into p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com