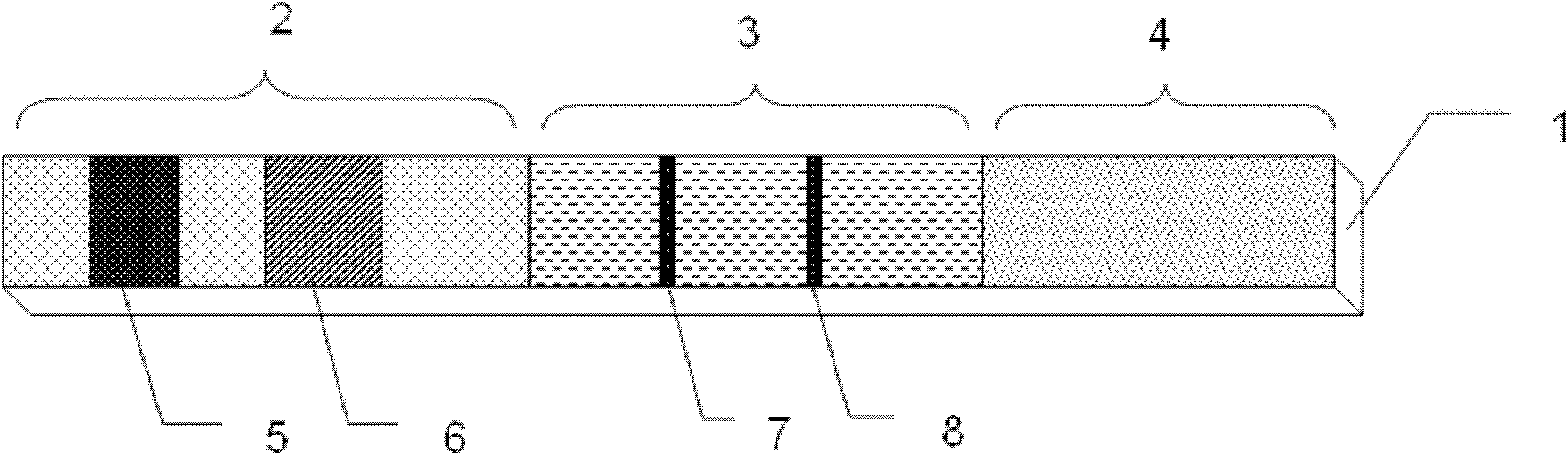
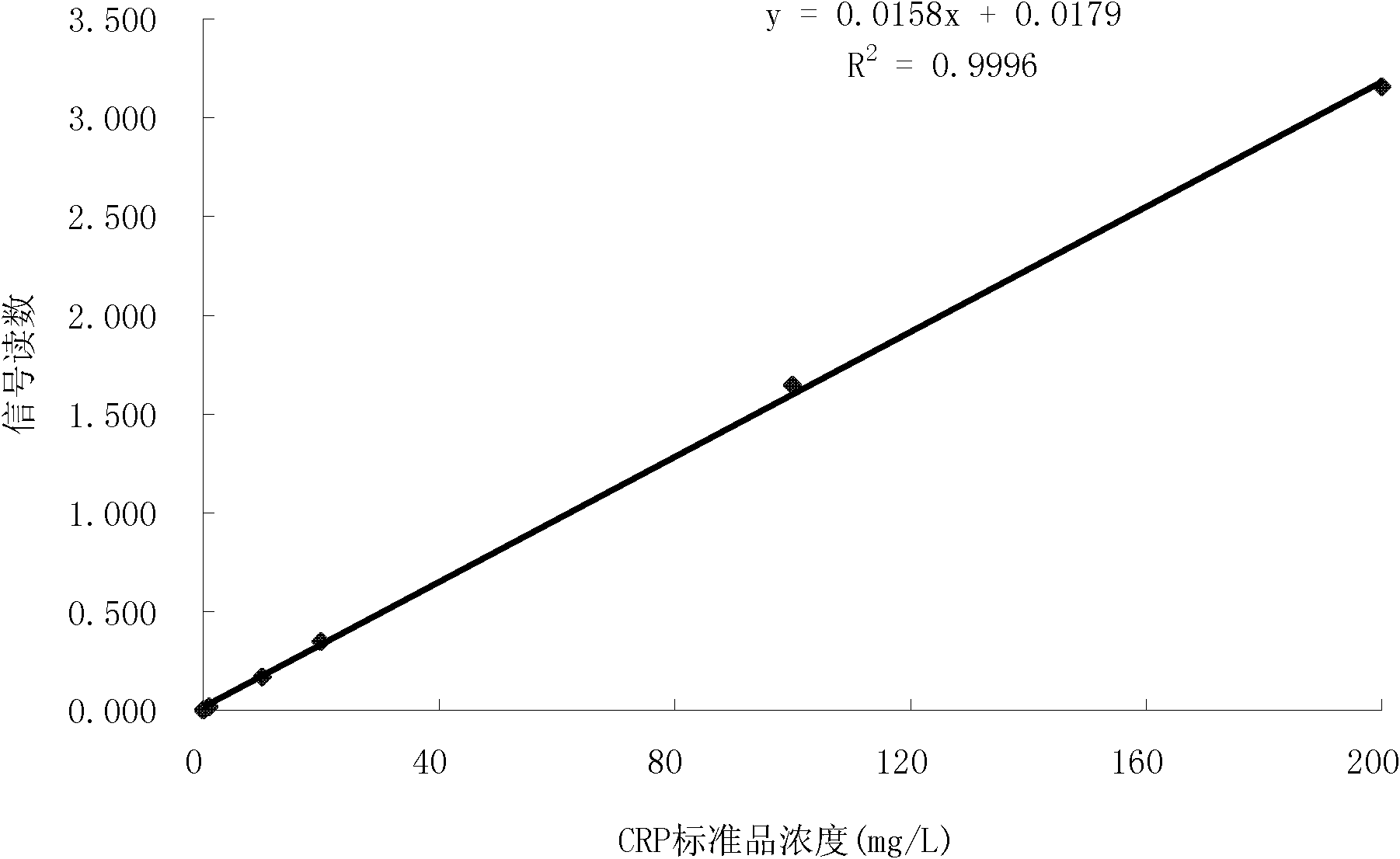Time-resolved immunoflourometric chromatographic test strip for quantitative detection as well as preparation method and application thereof
A technology for time-resolved fluorescence and detection of test paper, which is applied in the direction of analytical materials, measuring devices, instruments, etc., can solve the problems of complex production process, high requirements for instruments and equipment, and difficulty in fixing antibodies, and achieve the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
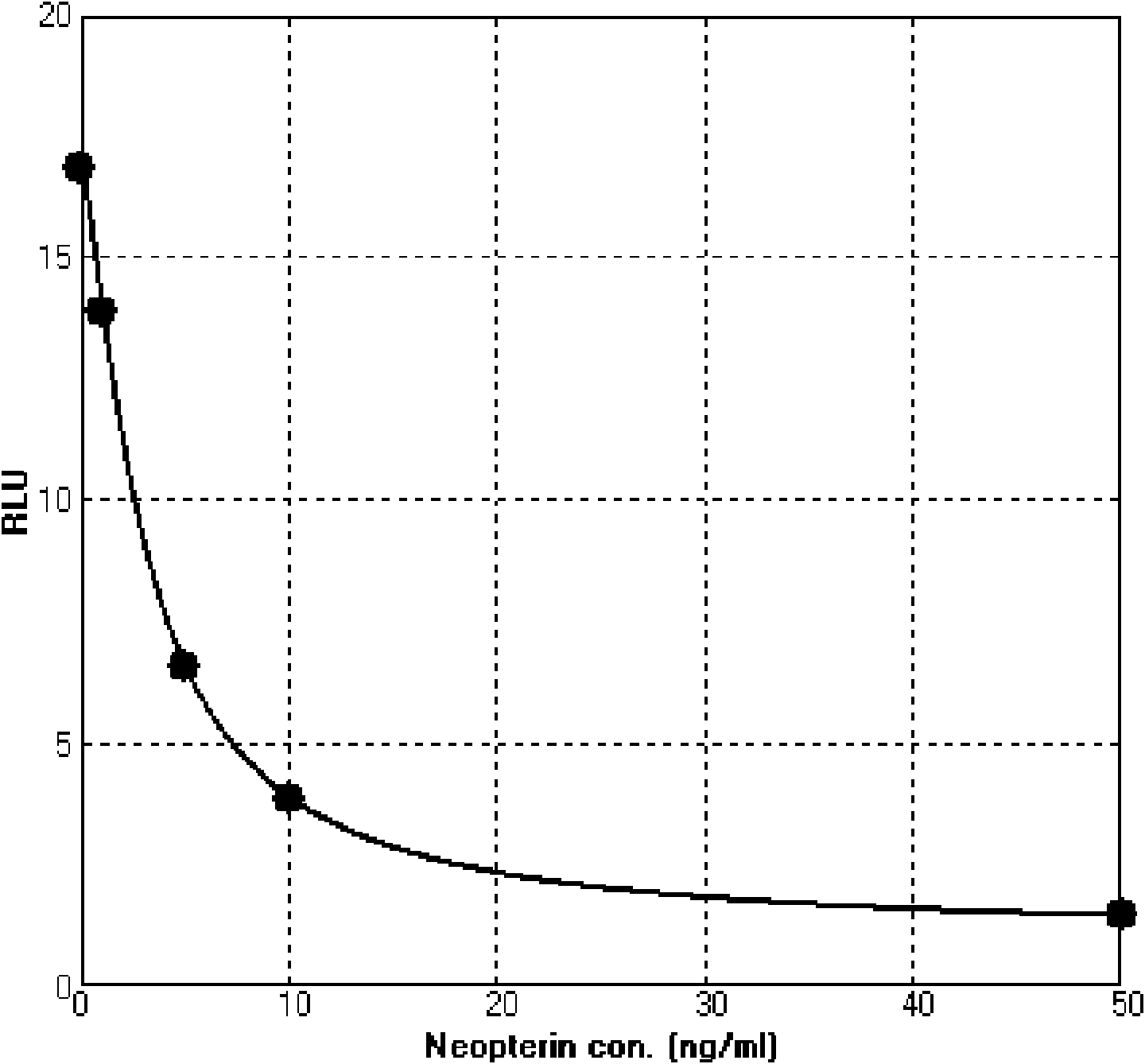
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of quality control microspheres coated with biotin-labeled γ-globulin (BGG)
[0042] (1) Preparation of biotinylated BGG
[0043] with 0.1M NaCNBH 3BGG (purchased from Pel-Freez Biological) was formulated into a 10mg / ml solution, and Biotin-X-X-NHS (N-hydroxysuccinimide modified biotin) was prepared using DMSO (dimethylformamide), manufacturer: SIGMA, product No.: B3295) solution to 16.172mg / ml, add the Biotin-X-X-NHS solution to the BGG solution according to the amount of 1mg antibody added to 5.4ul Biotin-X-X-NHS, mix well and place overnight at 4°C. Dialysis was used to remove free unreacted biotin, and the dialysate was biotin-labeled antibody dialysate buffer (0.1M Tris, 0.3MNaCl, 0.005MEDTA-Na-2H 2 O, pH8.0). After dialysis, the protein concentration was determined by BCA method.
[0044] (2) The luminescent microspheres coated with aldehyde group modified by the above-mentioned labeled protein
[0045] Add 6.4 μl of 20% Tween-20 solutio...
Embodiment 2
[0048] Example 2 Preparation of quality control microspheres coated with biotin-labeled bovine serum albumin (BSA)
[0049] (1) Preparation of biotin-labeled bovine serum albumin (BSA)
[0050] with 0.1M NaCNBH 3 Bovine serum albumin (purchased from EQUITECH-BIO INC) was formulated into a 10mg / ml solution, and Biotin-X-X-NHS (N-hydroxysuccinimide modified biotin, manufacturer : SIGMA, product number: B3295) solution to 16.172mg / ml, add Biotin-X-X-NHS solution to the calf serum albumin solution according to the amount of 1mg antibody added to 13.5ul Biotin-X-X-NHS, mix well and store at 4°C Set aside overnight. Dialysis was used to remove free unreacted biotin, and the dialysate was biotin-labeled antibody dialysate buffer (0.1M Tris, 0.3M NaCl, 0.005M EDTA-Na-2H 2 O, pH 8.0). After dialysis, the protein concentration was determined by BCA method.
[0051] (2) Use the above-mentioned labeled protein to coat the aldehyde-modified fluorescent microspheres
[0052] Coating m...
Embodiment 3
[0056] Example 3 C-reactive protein (CRP) time-resolved fluorescent immunoassay strip
[0057] 1. Preparation of test strip components:
[0058] (1) Preparation of quality control microspheres
[0059] Refer to Example 1 or Example 2 for the preparation method of quality control microspheres.
[0060] (2) Preparation of detection microspheres
[0061] Add 6.4 μl of 20% Tween-20 solution and 2 mg C-reactive protein monoclonal antibody (Boyang Biotechnology (Shanghai) Co., Ltd.) to 10 mg of aldehyde-modified fluorescent microspheres (Boyang Biotechnology (Shanghai) Co., Ltd.) and 16 μl of NaCNBH 3 (25mg / ml, 0.05M pH6.0 MES buffer preparation, ready to use), add 0.1M pH6.0MES to a total volume of 400μl, and incubate at 37° in the dark for 48h. Add 40 μl of Gly solution (75 mg / ml, prepared in 0.05 M MES buffer solution with pH 6.0), and rotate for 2 hours at 37° in the dark. Add 250 μl of N, O-bistrimethylsilylacetamide (200 mg / ml, prepared in 0.05 M MES buffer, pH 6.0) solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com