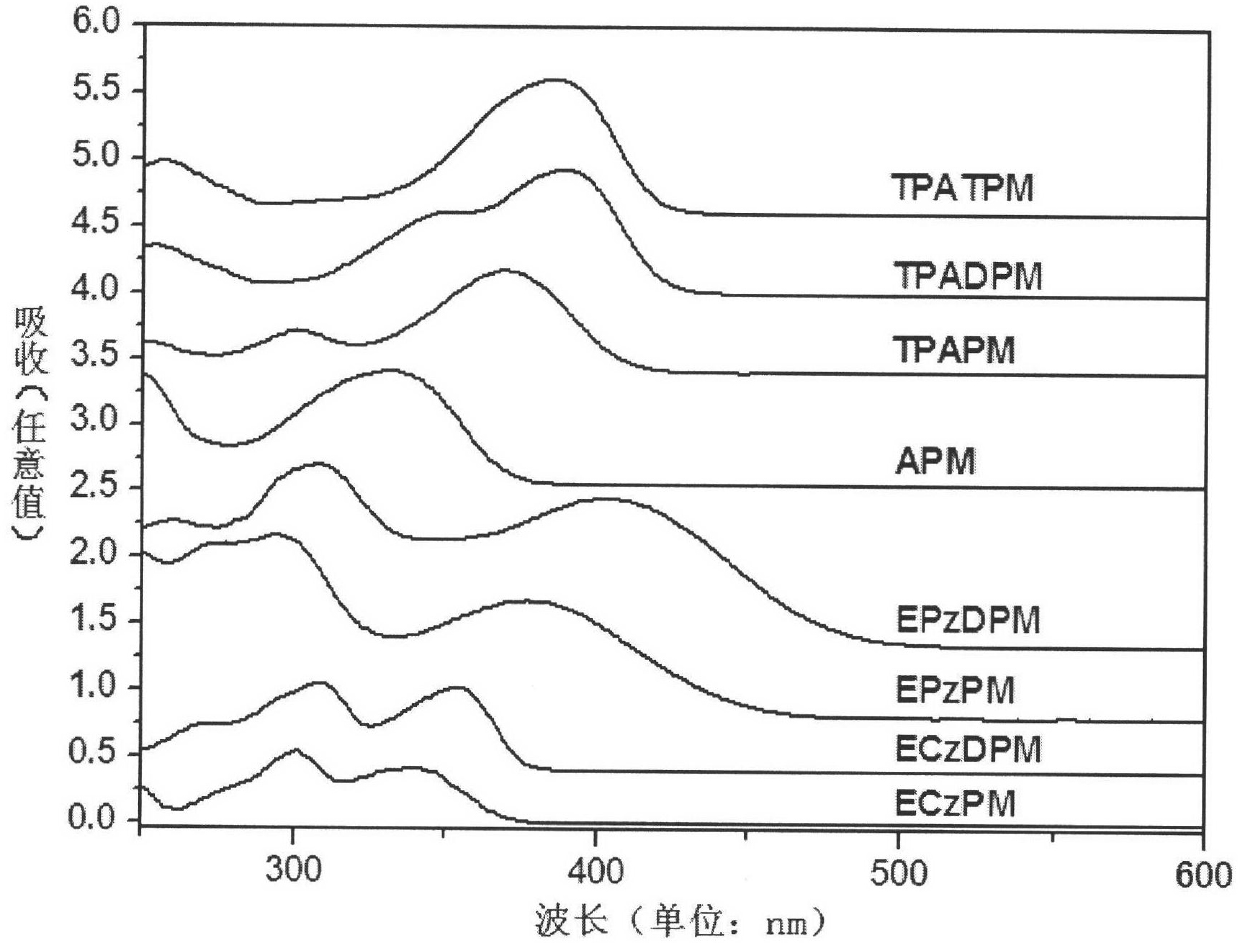
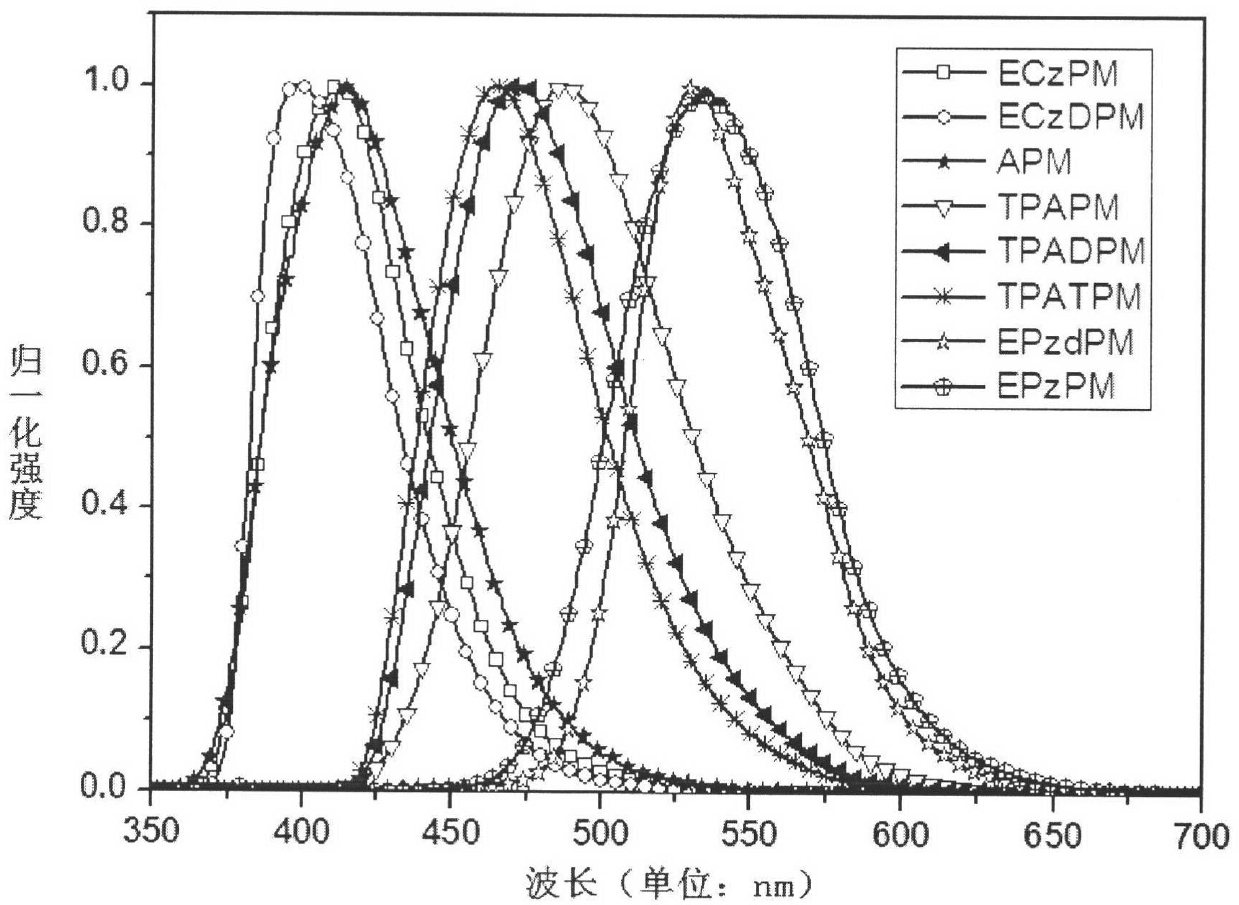
4-aryl pyrimidine or 4-heterocyclic aryl pyrimidine compound luminescent material and preparation method thereof
A technology of heterocyclic arylpyrimidine and arylpyrimidine, which is applied in the field of optoelectronic materials and can solve the problems that the efficiency cannot reach flexible large-area display screens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 2.3730g (10mmol) 3-acetyl-N-ethylcarbazole, 1.5416g (20mmol) ammonium acetate and 0.1363g (1mmol) zinc chloride to the two-neck flask, seal the device and protect the reaction system with nitrogen. Inject 5ml (30mmol) of triethyl orthoformate and 10ml (94mmol) of toluene under the protection of nitrogen. The reaction mixture was heated to 100 °C and stirring was continued at this temperature for 24 h. After cooling to room temperature, slowly add saturated sodium carbonate solution, stir for 30 min, and extract with dichloromethane. The organic phase was collected and dried with anhydrous sodium sulfate, concentrated, and separated by column chromatography. The obtained white solid was 4-pyrimidine luminescent material substituted with N-ethylcarbazole (ECzPM), and the yield was 20.5%. 1 HNMR (400MHz, CDCl 3 ): δ9.27(s, 1H), 8.90(s, 1H), 8.72-8.71(d, 1H), 8.22-8.18(t, 2H), 7.81-7.80(d, 1H), 7.54-7.43(m , 3H), 7.32-7.28 (m, 3H), 4.41-4.36 (q, 2H), 1.48-1.46 (t, 3H...
Embodiment 2
[0030] Add 1.3950g (5mmol) 3,6-diacetyl-N-ethylcarbazole, 1.9270g (25mmol) ammonium acetate and 0.2045g (1.5mmol) zinc chloride to the two-necked flask, seal the device and protect the reaction system with nitrogen . Under nitrogen protection, 5ml (46mmol) of trimethyl orthoformate and 12ml (113mmol) of toluene were injected. The reaction mixture was heated to 95 °C and stirring was continued at this temperature for 12 h. After cooling to room temperature, slowly add saturated sodium carbonate solution, stir for 40 min, and extract with dichloromethane. The organic phase was collected and dried with anhydrous sodium sulfate, concentrated, and separated by column chromatography. The obtained white solid was N-ethylcarbazole-substituted bis-4-pyrimidine luminescent material (ECzDPM), with a yield of 14.5%. 1 H NMR (400MHz, CDCl 3 ): δ9.27(s, 2H), 8.97(s, 2H), 8.75-8.74(d, 2H), 8.28-8.25(d, 2H), 7.83-7.82(d, 2H), 7.52-7.50(d , 2H), 4.44-4.39 (q, 2H), 1.51-1.47 (m, 3H). 13 C ...
Embodiment 3
[0032] Add 2.6900g (10mmol) of 3-acetyl-N-ethylphenothiazine, 2.3124g (30mmol) of ammonium acetate and 0.6818g (5mmol) of zinc chloride into the two-necked flask, seal the device and protect the reaction system with nitrogen. Inject 10ml (60mmol) of triethyl orthoformate and 5ml (47mmol) of toluene under the protection of nitrogen. The reaction mixture was heated to 120 °C, and stirring was continued at this temperature for 72 h. After cooling to room temperature, slowly add saturated sodium carbonate solution, stir for 20 min, and extract with dichloromethane. The organic phase was collected and dried with anhydrous sodium sulfate, concentrated, and separated by column chromatography. The obtained yellow viscous material was 4-pyrimidine luminescent material (EPzPM) substituted with N-ethylphenothiazine, and the yield was 13.5%. 1 H NMR (400MHz, CDCl 3 ): δ9.19(s, 1H), 8.70-8.69(d, 1H), 7.92-7.89(d, 1H), 7.85(s, 1H), 7.62-7.60(d, 1H), 7.18-7.12(m , 2H), 6.96-6.88 (m, 3H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com