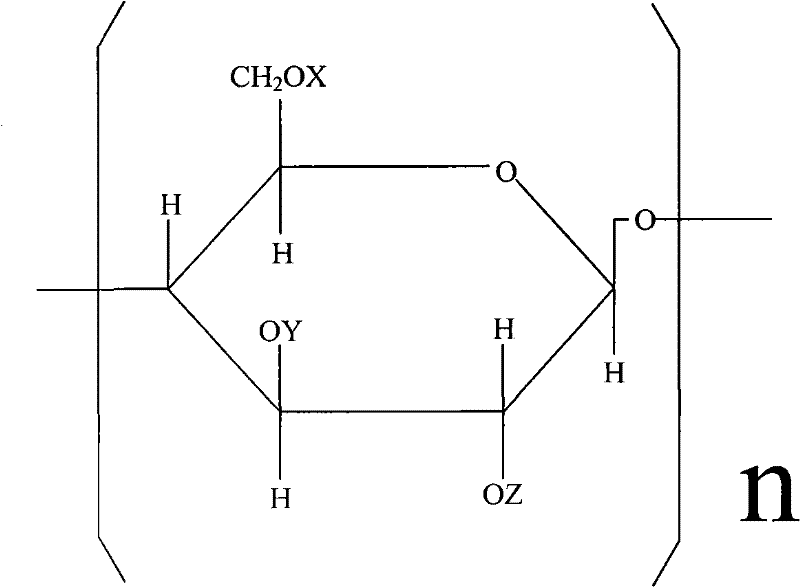
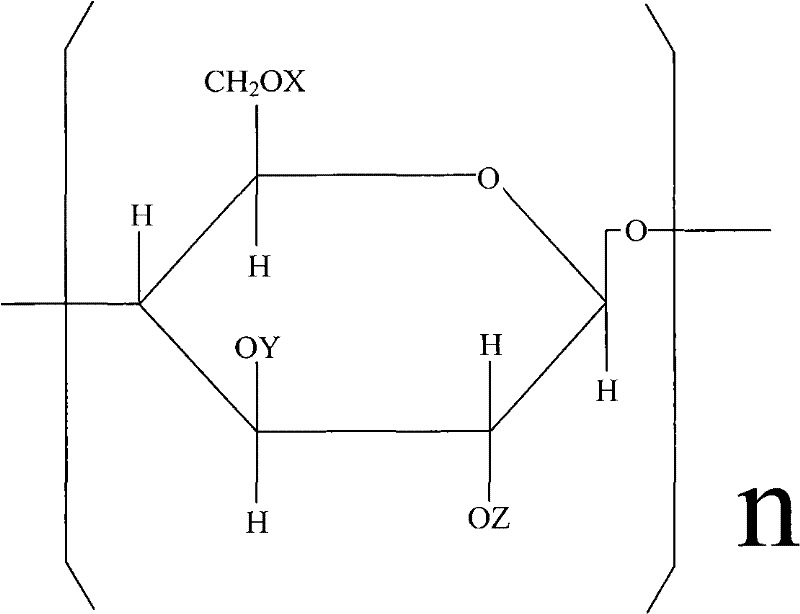
Carboxymethyl hydroxyethyl modified cotton fiber hemostyptic fabric and preparation method thereof
A carboxymethyl hydroxyethyl, cotton fiber fabric technology, applied in the field of polymer chemistry, can solve problems such as inconvenient operation, and achieve the effect of eliminating coagulation or poor absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Put 20.5 g of pure cotton degreasing gauze in 20% NaOH aqueous solution, basify for 2 hours at a temperature of t=18° C., and centrifugally deliquify until the weight of the gauze containing lye is 36.9 g. Arrange the alkalized yarn in a reactor, add 400ml of 95% isopropanol, vacuumize to -0.08Mpa, inhale 18.6ml of ethylene oxide several times, and react at 20-25°C for 10 hours. Then add 20.5 g of monochloroacetic acid, stir to dissolve, add 21.6 ml of 40% NaOH aqueous solution at the same time, continue to stir and react for 10 hours, and the reaction temperature t=20~30°C. After the reaction, adjust the pH to 7.1 with hydrochloric acid, and then wash the gauze with 80% isopropanol until the gauze contains NaCl below 1%. Finally, the yarn is dried in a drying oven at a drying temperature of <80°C. Obtain 33.5 g of carboxymethyl hydroxyethyl modified cotton fiber gauze.
Embodiment 2
[0028] Pure cotton degreasing gauze 26.5g, put in 25% NaOH aqueous solution, alkalization temperature t=20 ℃, time 1 hour. Centrifuge and deliquify until the gauze containing lye weighs 53g. Arrange the alkalized yarn in the reactor, add 450ml of 95% isopropanol, vacuumize to -0.08Mpa, add 30ml of ethylene oxide in several times, the reaction temperature is 25°C, and the reaction time is 8 hours. Then 15.9 g of monochloroacetic acid was added, stirred to dissolve, and 16.7 ml of 40% NaOH aqueous solution was added at the same time, and reacted at 20-25° C. for 12 hours. Then adjust the pH to 7 with hydrochloric acid, and wash the gauze with 80% isopropanol until the NaCl content in the gauze is below 1%. Finally, place the yarn in a drying oven to dry at 65°C. Obtain carboxymethyl hydroxyethyl modified cotton fiber gauze 46.7g.
[0029] Tests have confirmed that the average hemostatic time for carboxymethyl hydroxyethyl modified cotton fiber hemostatic fabric on surgical wo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com