Pentadienone-containing 4-substituted quinazoline derivative, preparation method and use thereof
A kind of technology of quinazoline and pentadienone, which is applied in the field of chemistry and achieves good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
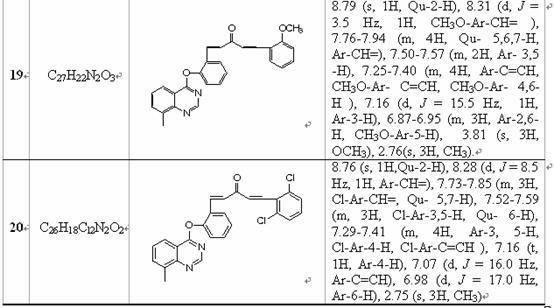
[0033] Example 1: Synthesis of 1-(4-fluorophenyl)-5-(2-(quinazolin-4-oxyl) 1,4-pentadien-3-one:
[0034] (1) Synthesis of quinazolin-4-one:
[0035] Add 13.7g (0.1mol) 2-aminobenzoic acid and 20mL (4.2mol) formamide in the 250mL there-necked flask equipped with a thermometer and a condenser tube, keep the temperature between 135-145°C for reaction, during this process, the system From light gray turbid liquid to yellow-brown clear liquid, a small amount of light gray solid appeared on the wall of the reactor after 2 hours of reaction, and the reaction was complete after 4.5 hours. Formamide was decomposed, and a large amount of light gray solids were precipitated. Naturally cooled, suction filtered, and dried to obtain 14.52 g of the product, which was recrystallized with absolute ethanol to obtain 13.15 g of light gray flaky crystals, with a yield of 89.8%.
[0036] (2) Synthesis of 4-chloroquinazoline:
[0037] Add 11.00g (76mmol) of 4-quinazolinone, 80mL thionyl chloride,...
Embodiment 2
[0044] Example 2: Synthesis of 1-(3-nitrophenyl)-5-(2-(quinazolin-4-oxyl) 1,4-pentadien-3-one:
[0045] (1) Synthesis of quinazolin-4-one:
[0046] (2) Synthesis of 4-chloroquinazoline:
[0047] (3) Synthesis of 4-(2-hydroxyphenyl)-3-buten-2-one:
[0048] (4) Synthesis of 4-(2-(quinazolin-4-oxyl)phenyl)-3-buten-2-one:
[0049] Above four steps are with embodiment 1 (1)-(4) step.
[0050] (5) Synthesis of 1-(3-nitrophenyl)-5-(2-(quinazolin-4-oxyl)1,4-pentadien-3-one:
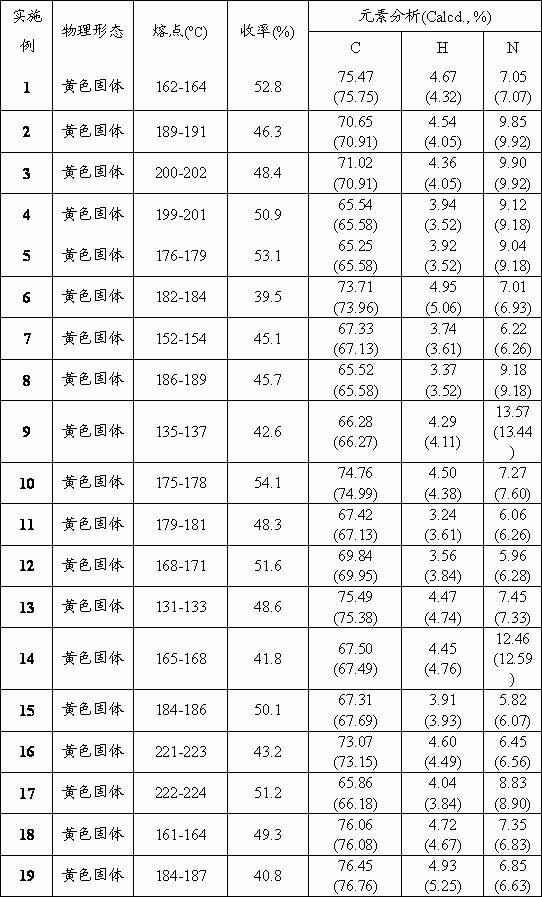
[0051] Synthesize as in Example 1 (5) step method and conditions. The difference is that 3-nitrobenzaldehyde (0.5 mmol, 0.08 g) was added, reacted for 2 hours, melting point 189-191 o C, the yield is 46.3%.
Embodiment 3
[0052] Example 3: Synthesis of 1-(4-nitrophenyl)-5-(2-(quinazolin-4-oxyl) 1,4-pentadien-3-one:
[0053] (1) Synthesis of quinazolin-4-one:
[0054] (2) Synthesis of 4-chloroquinazoline:
[0055] (3) Synthesis of 4-(2-hydroxyphenyl)-3-buten-2-one:
[0056] (4) Synthesis of 4-(2-(quinazolin-4-oxyl)phenyl)-3-buten-2-one:
[0057] Above four steps are with embodiment 1 (1)-(4) step.
[0058] (5) Synthesis of 1-(4-nitrophenyl)-5-(2-(quinazolin-4-oxyl)1,4-pentadien-3-one:
[0059] Synthesize as in Example 1 (5) step method and conditions. The difference is that 4-nitrobenzaldehyde (0.5 mmol, 0.08 g) was added, reacted for 2.5 h, melting point 200-202 o C, the yield is 48.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com