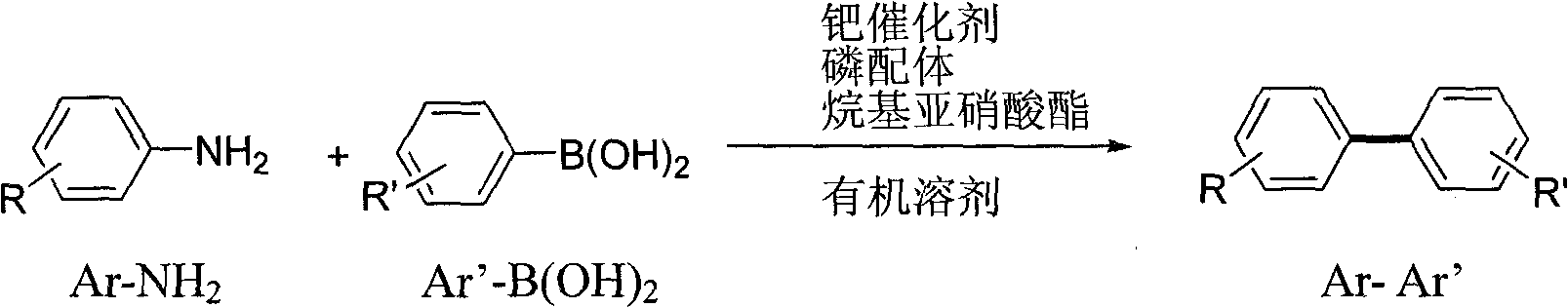
Method for preparing aromatic cross-coupled compound
A cross-coupling and compound technology, which is applied in the field of preparation of aromatic cross-coupling compounds, can solve problems such as difficult access to aryl diazonium salts, limited application of coupling reactions, sensitivity to heat and light, and achieves low cost and low substrate Stable, well tolerated effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
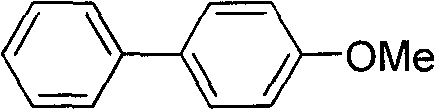
[0029] Synthesis of 4-methoxybiphenyl
[0030] Add 182mg (i.e. 1.2mmol) p-methoxyphenylboronic acid, 23mg (i.e. 0.025mmol) Pd 2 (dba) 3 , 35mg (ie 0.15mmol) P (furyl) 3 , 2ml DMF. Then weigh 93mg (i.e. 1mmol) of aniline and 113mg (i.e. 1.1mmol) of tert-butyl nitrite in a long tube type reaction bottle, add 2ml of DMF, stir and inject into the Schlenk bottle with a needle, and the system is heated at 80°C React for 8 hours, concentrate after the reaction, and use petroleum ether as the eluent for column chromatography purification to obtain 4-methoxybiphenyl, whose structure is shown in the following formula:
[0031]
[0032] The compound is a white solid with a yield of 81%, and its NMR data are as follows:
[0033] 1 H NMR (400MHz, CDCl 3 )δ7.56~7.51(m, 4H), 7.43~7.39(m, 2H), 7.31~7.28(m, 1H), 6.99~6.96(m, 2H), 3.84(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ 159.1, 140.8, 133.7, 128.7, 128.1, 126.7, 126.6, 114.2, 55.3.
Embodiment 2
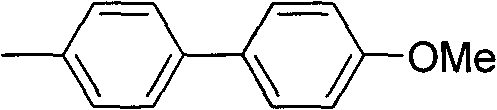
[0035] Synthesis of 4-methyl 4'-methoxybiphenyl
[0036] Add 182mg (i.e. 1.2mmol) p-methoxyphenylboronic acid, 23mg (i.e. 0.025mmol) Pd 2 (dba) 3 , 35mg (ie 0.15mmol) P (furyl) 3 , 2ml DMF. Then take by weighing 107mg (i.e. 1mmol) p-toluidine and 113mg (i.e. 1.1mmol) tert-butyl nitrite in the long tube type reaction bottle, add 2ml DMF, inject in the Schlenk bottle with needle after stirring, system is at 80 React at ℃ for 8 hours, concentrate after reaction, and use petroleum ether as eluent for column chromatography purification to obtain 4-methyl-4'-methoxybiphenyl, whose structure is shown in the following formula:
[0037]
[0038] The compound is a yellow solid with a yield of 77%, and its NMR data are as follows:
[0039] 1 H NMR (400MHz, CDCl 3 )δ7.50(d, 2H, J=8.8Hz), 7.44(d, 2H, J=8.1Hz), 7.22(d, 2H, J=7.9Hz), 6.96(d, 2H, J=8.8Hz) , 3.83(s, 3H), 2.34(s, 3H); 13 C NMR (100MHz, CDCl 3 ) 158.9, 137.9, 136.3, 133.7, 129.4, 127.9, 126.5, 114.1, 55.3, 21.0.
Embodiment 3
[0041] Synthesis of 4-Chloro4'-Methoxybiphenyl
[0042] Add 182mg (i.e. 1.2mmol) p-methoxyphenylboronic acid, 23mg (i.e. 0.025mmol) Pd 2 (dba) 3 , 35mg (ie 0.15mmol) P (furyl) 3 , 2ml DMF. Then take by weighing 128mg (i.e. 1mmol) p-chloroaniline and 113mg (i.e. 1.1mmol) tert-butyl nitrite in the long tube type reaction flask, add 2ml DMF, inject in the Schlenk bottle with needle after stirring, system is at 80 React at ℃ for 8 hours, concentrate after the reaction, and use petroleum ether as the eluent for column chromatography purification to obtain 4-chloro-4'-methoxybiphenyl, whose structure is shown in the following formula:
[0043]
[0044] The compound is a yellow solid with a yield of 75%, and its NMR data are as follows:
[0045] 1 H NMR (400MHz, CDCl 3 )δ7.48(d, 2H, J=6.3Hz), 7.46(d, 2H, J=6.1Hz), 7.37(d, 2H, J=8.7Hz), 6.97(d, 2H, J=8.9Hz) , 3.84(s, 3H); 13 C NMR (100MHz, CDCl 3 ) δ 159.3, 139.2, 132.6, 132.4, 128.8, 127.9, 127.9, 114.3, 55.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com