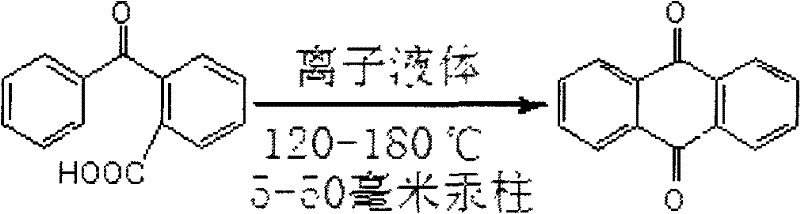
Method for synthesizing anthraquinone
A synthesis method and anthraquinone technology are applied in the field of organic compound preparation, can solve problems such as unreported synthetic application of anthraquinone, and achieve the effects of low cost, simplified post-processing steps, and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 5g (35mmol) of dimethylamine sulfate and 1.13g (5mmol) of o-benzoylbenzoic acid into a flask, install a condenser tube, and connect it to the decompression system. Then, under the pressure of 5mmHg, it was heated to 120° C., and after 5 hours of reaction, the heating was stopped and the pressure was returned to atmospheric pressure. 0.84 g of product anthraquinone was collected from the condenser tube, and the yield was 80.8%.
Embodiment 2
[0017] Add 5g (about 29mmol) of diethylamine sulfate and 1.13g (5mmol) of o-benzoylbenzoic acid into a flask, install a condenser tube, and connect it to the decompression system. Then, under the pressure of 10 mmHg, heat to 130° C., and after 5 hours of reaction, stop heating and return the pressure to atmospheric pressure. 0.80 g of product anthraquinone was collected from the condenser tube, and the yield was 76.9%.
Embodiment 3
[0019] Add 5g (25mmol) of triethylamine sulfate and 1.13g (5mmol) of o-benzoylbenzoic acid into a flask, install a condenser tube, and connect it to the decompression system. Then, under the pressure of 25 mmHg, heat to 150° C., after 3 hours of reaction, stop heating and return the pressure to atmospheric pressure. 0.75 g of product anthraquinone was collected from the condenser, and the yield was 72.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com