New S-omeprazole salts
A technology for omeprazole potassium salt and omeprazole is applied in the field of novel S-omeprazole salt, can solve problems such as poor crystallinity, and achieve the effects of easy processing and storage, and easy large-scale preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
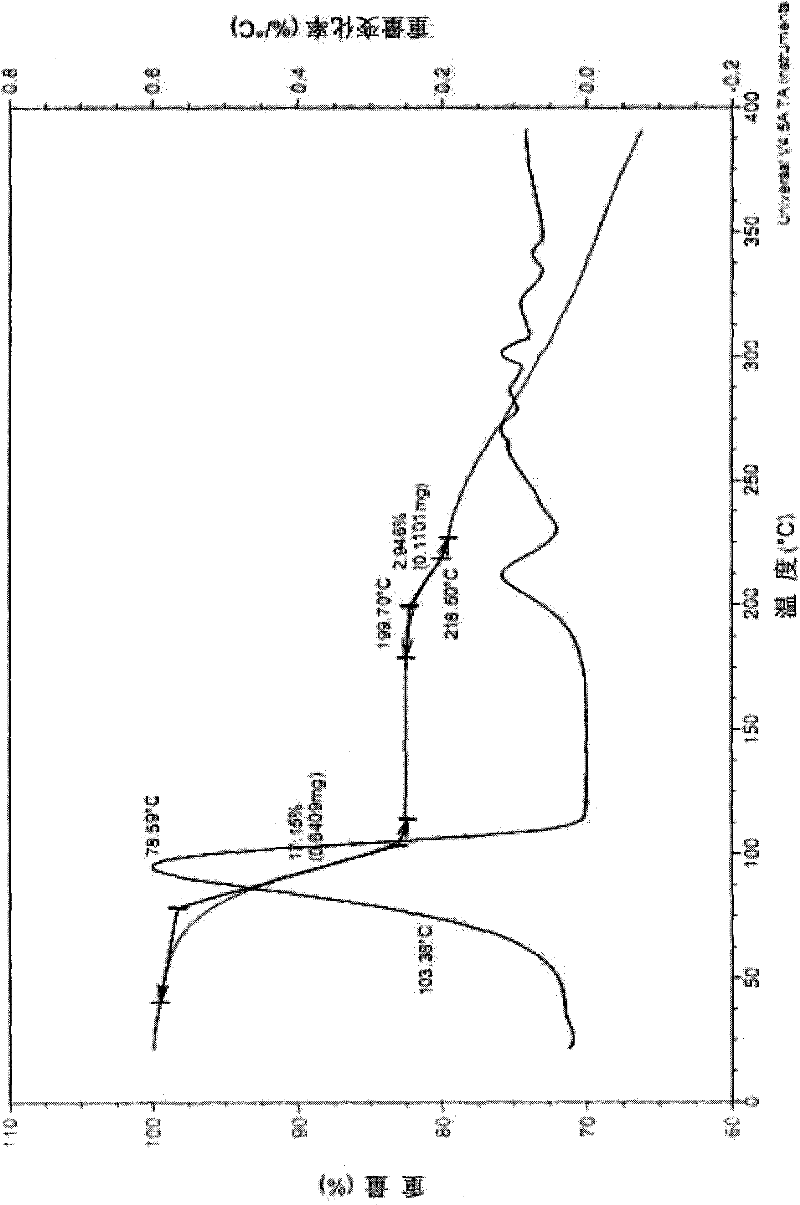
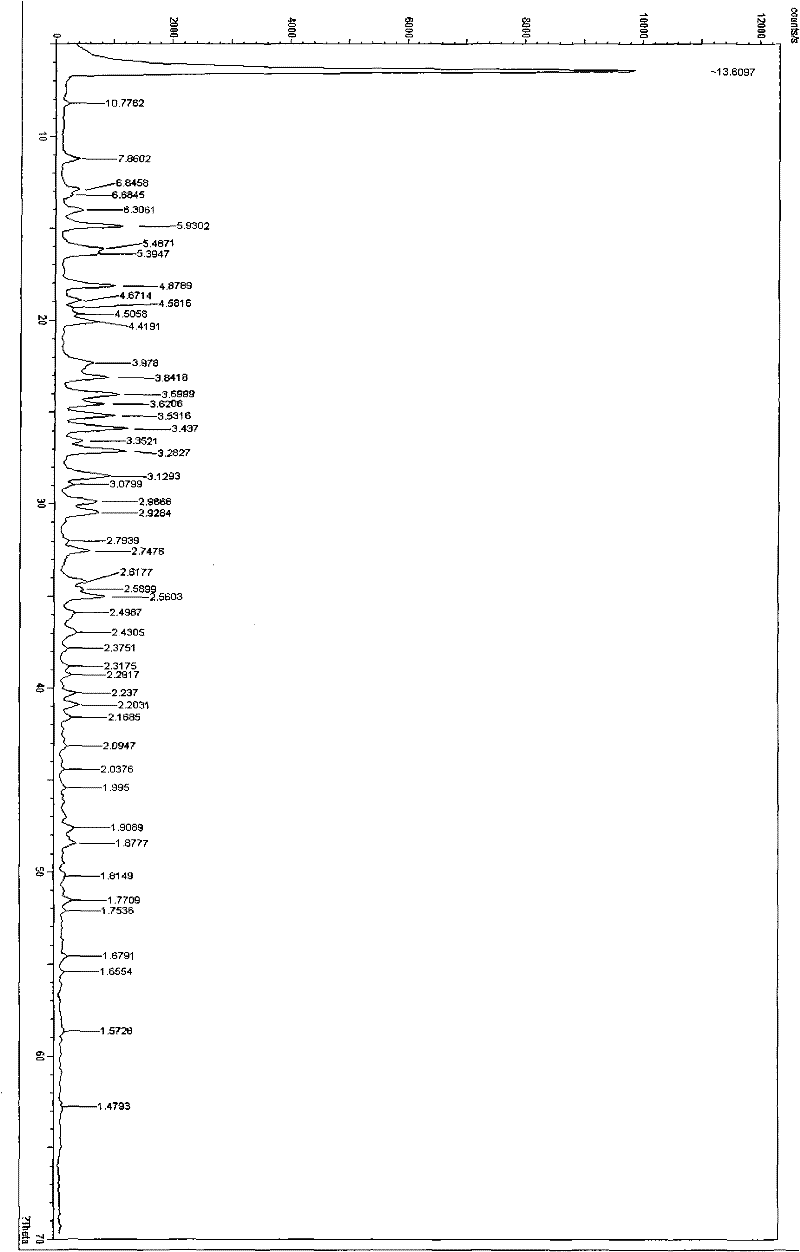
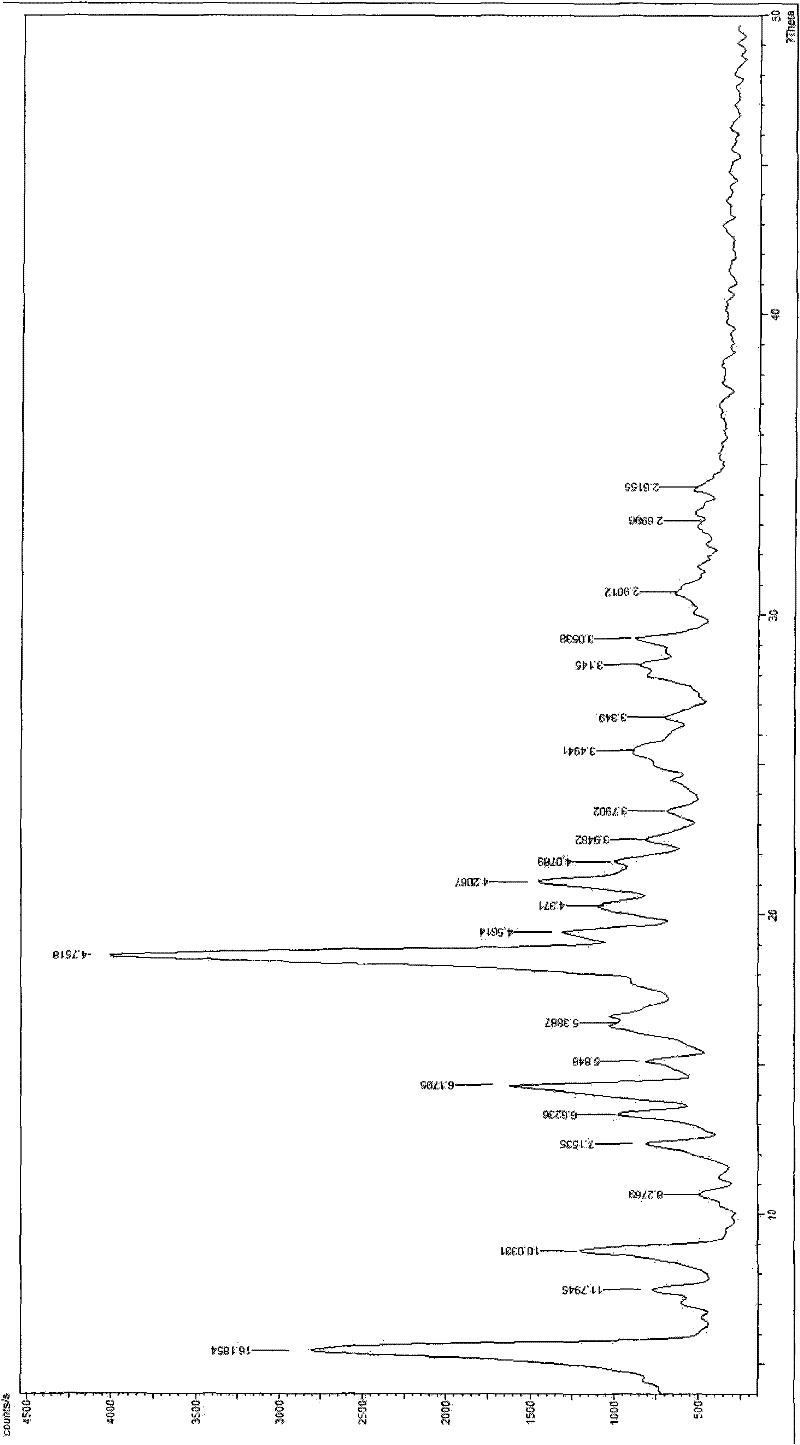
Image
Examples
Embodiment Construction
[0042] Example 1: Preparation of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole
[0043] 0.348 g (1.5 mmol) of D-di-n-propionamide tartrate was added to the reactor, 7.5 ml of toluene was added, the temperature was raised to 60°C under stirring, and 0.225 ml (0.75 mmol) of Ti(Oi-Pr) was added 4 After 60 minutes, 0.0068 ml (0.375 mmol) of water was added and stirred for another 60 minutes. Cool down to 30°C, add 0.822 g (2.5 mmol) omeprazole precursor thioether, after stirring for 10 minutes, add 0.5 ml (2.7 mmol) 80% CHP dropwise, keep the reaction for 5 hours, the product is not separated, take A small amount of reaction solution is measured by HPLC and enantioselectivity is 80%, by 1 The yield calculated by H NMR internal standard method was 50%.
[0044] Example 2: Preparation of 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole
[0045] 0.348 g (1.5 mmol) of D-di-n-propionamide tartrate was added to the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com