Method for quickly synthesizing MOFs nanoparticles
A nano-particle, rapid technology, applied in chemical instruments and methods, alkali metal compounds, zinc organic compounds, etc., can solve the problems of uneven particle size distribution of concentration products, long synthesis cycle, difficult to control the shape, etc., to shorten the synthesis time The effects of time, mild reaction conditions, and narrow particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
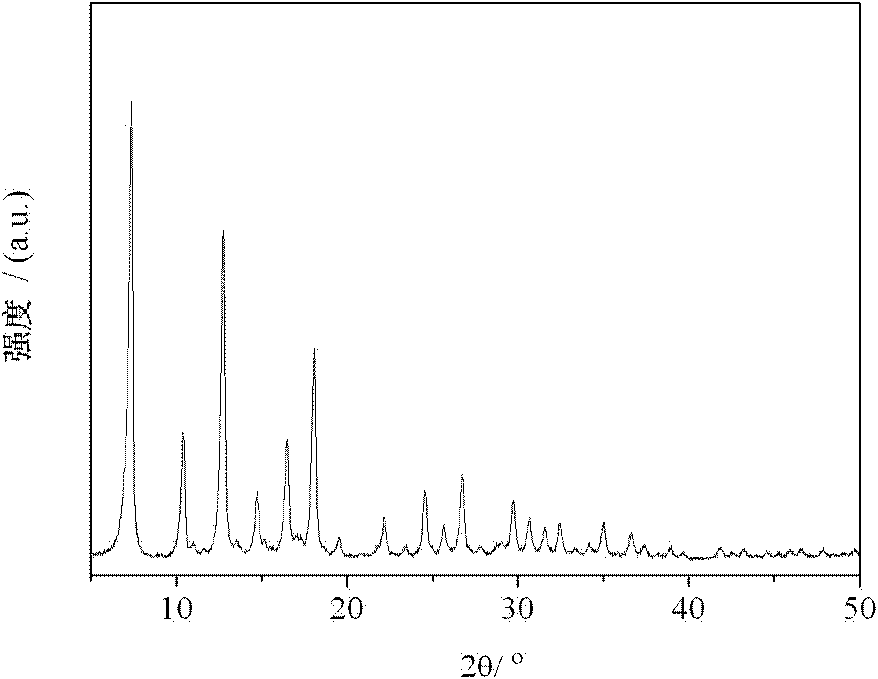
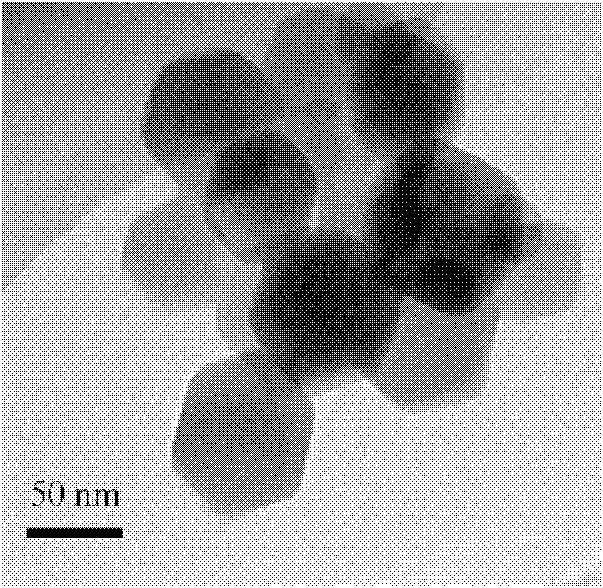
Image
Examples
Embodiment 1
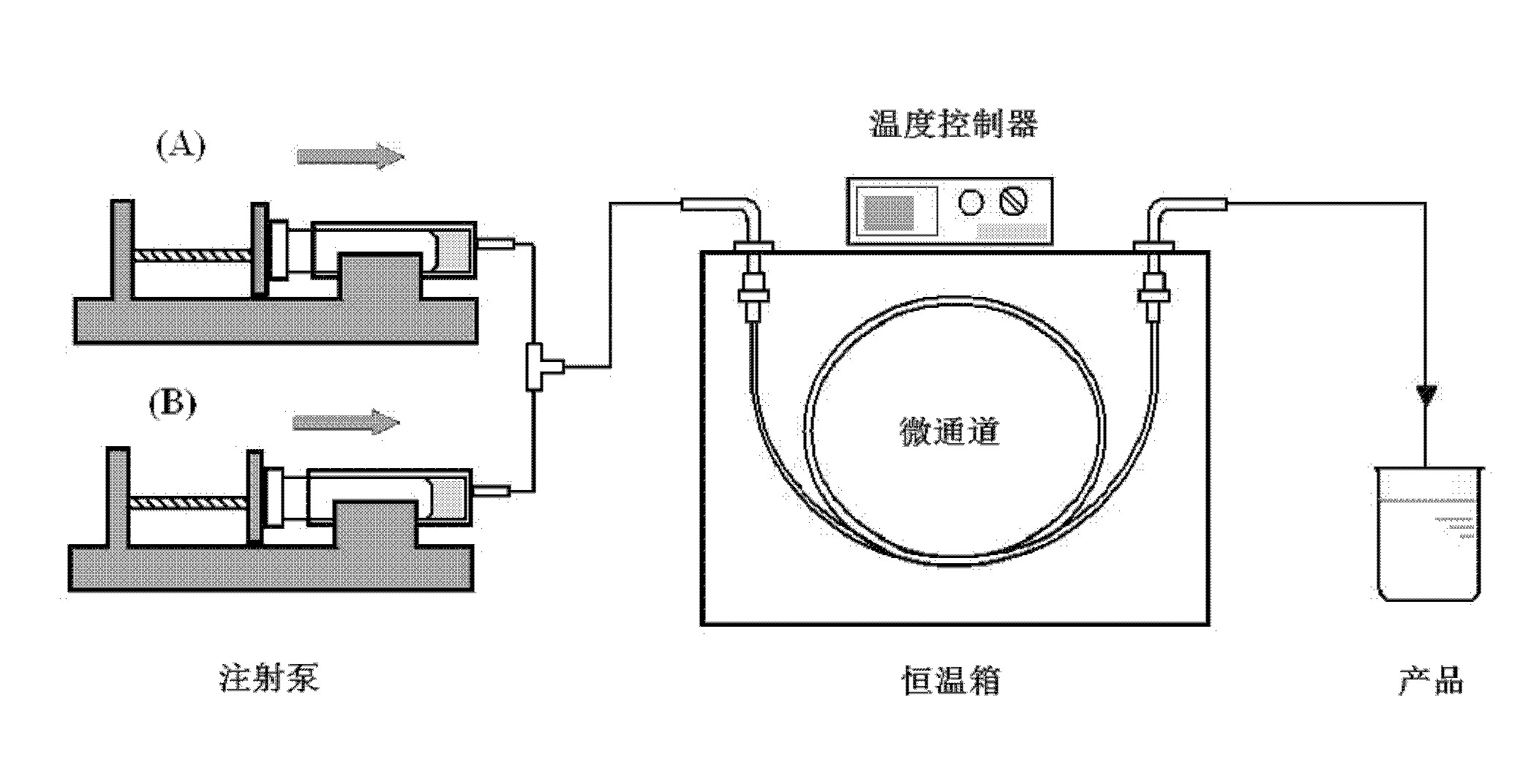
[0025] 1. Microchannel pretreatment: select a capillary quartz tube with an inner diameter of 0.5mm and a length of 1m as the microchannel, wash the capillary quartz tube with 0.1mol / L nitric acid, 0.1mol / L sodium hydroxide solution and acetone respectively, and finally Rinse with deionized water before use.
[0026] 2. Preparation of precursor solution
[0027] (1) Precursor A: Weigh 5.078g of methylimidazole and dissolve it in 100ml of DMF to prepare a 0.6M methylimidazole DMF solution.
[0028] (2) Precursor solution B: Weigh 5.95g Zn(NO 3 ) 2 ·6H 2 O was dissolved in 100ml DMF to prepare 0.2M Zn(NO 3 ) 2 ·6H 2 O DMF solution.
[0029] 3. The precursor solution is synthesized in the microchannel
[0030] Put the two precursors into two syringes respectively, pump them into the three-way mixer with a syringe pump and mix them completely before entering the capillary quartz tube. The synthesis temperature is controlled at 80 °C, and the residence time of the precursor...
Embodiment 2
[0033] 1. Microchannel pretreatment: select a capillary quartz tube with an inner diameter of 1mm and a length of 2.5m as the microchannel, wash the capillary quartz tube with 0.1mol / L nitric acid, 0.1mol / L sodium hydroxide solution and acetone respectively, and finally Rinse with deionized water before use.
[0034] 2. Preparation of precursor solution
[0035] (1) Precursor A: Weigh 5.078g of methylimidazole and dissolve it in 100ml of DMF to prepare a 0.6M methylimidazole DMF solution.
[0036] (2) Precursor solution B: Weigh 5.95g Zn(NO 3 ) 2 ·6H 2 O was dissolved in 100ml DMF to prepare 0.2M Zn(NO 3 ) 2 ·6H 2 O DMF solution.
[0037] 3. The precursor solution is synthesized in the microchannel
[0038] Put the two precursors into two syringes respectively, pump them into the three-way mixer with a syringe pump and mix them completely before entering the capillary quartz tube. The synthesis temperature is controlled at 100 ° C, and the residence time of the precurs...
Embodiment 3
[0041] 1. Microchannel pretreatment: select a capillary quartz tube with an inner diameter of 0.75mm and a length of 1.5m as the microchannel, wash the capillary quartz tube with 0.1mol / L nitric acid, 0.1mol / L sodium hydroxide solution and acetone respectively, Finally, rinse with deionized water before use.
[0042] 2. Preparation of precursor solution
[0043] (1) Precursor A: Weigh 5.078g of methylimidazole and dissolve it in 100ml of DMF to prepare a 0.6M methylimidazole DMF solution.
[0044] (2) Precursor solution B: Weigh 5.95g Zn(NO 3 ) 2 ·6H 2 O was dissolved in 100ml DMF to prepare 0.2M Zn(NO 3 ) 2 ·6H 2 O DMF solution.
[0045] 3. The precursor solution is synthesized in the microchannel
[0046] Put the two precursors into two syringes respectively, pump them into the three-way mixer with a syringe pump and mix them completely before entering the capillary quartz tube. The synthesis temperature is controlled at 90 ° C, and the residence time of the precurso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com