Preparation method of serial antibacterial peptide Alloferon-1 and application of rAlloferon-1-K
A technology of antibacterial peptides and tandems, which is applied in the preparation of tandem antibacterial peptide Alloferon-1 and the application field of rAlloferon-1-K, can solve the problems of low natural expression yield and difficulty in meeting industrialization requirements, and is conducive to popularization and application , the effect of reducing the preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
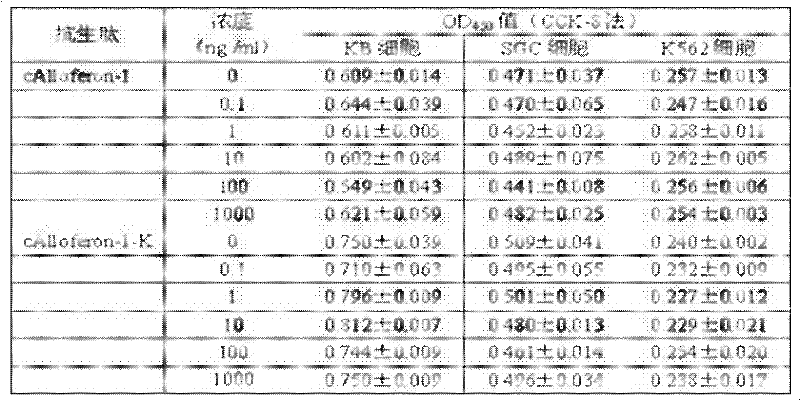
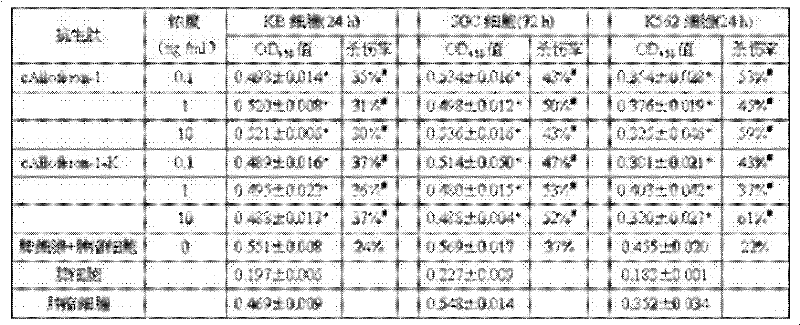
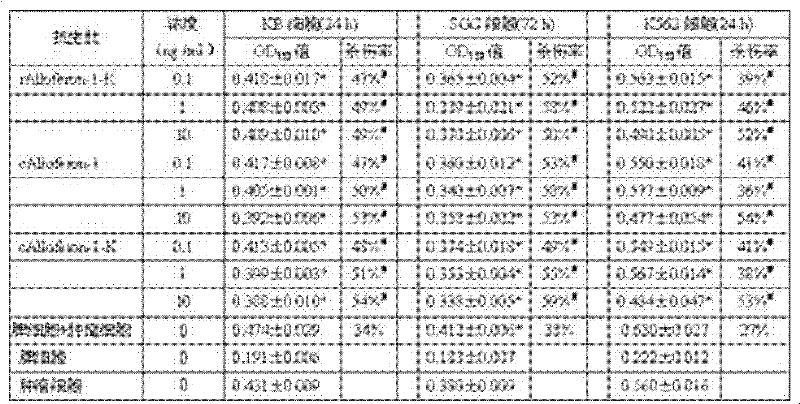
[0024] The preparation method of the tandem antimicrobial peptide Alloferon-1 is:
[0025] 1) Obtain the amino acid sequence of natural Alloferon-1 from GenBank: N-His Gly Val Ser Gly His Gly Gln His Gly Val His Gly-C (Alloferon-1 sequence does not contain arginine and lysine);
[0026] 2) Design a pair of non-mirror-symmetrical single-stranded DNA with complementary cohesive ends; according to the reported amino acid sequence of Alloferon-1 and the codon preference of E. coli, while considering the isolation of Alloferon-1 expressed in tandem, the positive strand sequence: 5'-phosphorylation -CATGGCGTGAGCGGCCATGGCCAGCATGGCGTGCATGGCAAA-3', a trypsin cleavage site lysine codon is set at the 3' end of the positive strand DNA Alloferon-1 gene, the negative strand sequence: 5'-phosphorylation-GCCATGGCCGCTCACGCCATGTTTGCCATGCACGCCATGCTG-3'. Negative-strand DNA interleaved with positive-strand; entrust Invitrogen to synthesize.
[0027] 3) Formation of double strands of the target p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com